Abstract
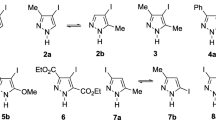
The 1H, 13C, and 15N chemical shifts of almost the whole series of N-benzyl azoles and benzazoles, with the exception of the unknown 1-benzyl-1H-pentazole (10) and the very unstable 2-benzyl-2H-isoindole (12), have been measured. In addition, the X-ray crystal structure of 1-benzyl-1H-indazole (14) was solved (monoclinic, space group P21/n), its geometry being very close to that used for the calculations. The absolute chemical shieldings were calculated at the gauge-independent atomic orbital (GIAO)/Becke, 3-parameter, Lee-Yang-Parr (B3LYP)/6-311++G(d,p) level and then transformed with very robust empirical equations into chemical shifts of the three nuclei showing an excellent agreement with the 313 experimental values.





Similar content being viewed by others
References
Comprehensive Heterocyclic Chemistry, three editions (I, II, III), Elsevier, Oxford, 1984, 1996, 2008
Katritzky AR, Lan X, Yang JZ, Denisko OV (1998). Chem Rev 98:409–548
Doiron J, Soultan AH, Richard R, Touré MM, Picot N, Richard R, Cuperlovic-Culf M, Robichaud GA, Touaibia M (2011). Eur J Med Chem 46:4010–4024
Begtrup M, Larson P (1990). Acta Chem Scand 44:1050–1057
Jones RG (1949). J Am Chem Soc 71:3994–4000
Jones RG, Ainsworth C (1954). J Am Chem Soc 77:1538–1540
Bulger PG, Cottrell IF, Cowden CJ, Davies AJ, Dolling UH (2000). Tetrahedron Lett 41:1297–1301
Ottoni O, Cruz R, Alves R (1998). Tetrahedron 54:13915–13928
Milen M, Grün A, Balint E, Dancso A, Keglevich G (2010). Synth Commun 40:2291–2301
Chen Q, Mao Z, Guo F, Liu X (2016). Tetrahedron Lett 57:3735–3738
Abenhaim D, Diez-Barra E, de la Hoz A, Loupy A, Sánchez-Migallón A (1994). Heterocycles 38:793–802
Nishi H, Kohno H, Kano T (1981). Bull Chem Soc Jpn 54:1897–1898
Sheldrick GM (2008). Acta Crystallogr A 64:112–122
Sheldrick GM (2015). Acta Crystallogr C 71:3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, JAK H, Puschmann H (2009). J Appl Crystallogr 42:339–341
Becke AD (1988). Phys Rev A 38:3098–3100
Becke AD (1993). J Chem Phys 98:5648–5652
Ditchfield R, Hehre WJ, Pople JA (1971). J Chem Phys 54:724–728
Frisch MJ, Pople JA, Binkley JS (1984). J Chem Phys 80:3265–3269
Sanz D, Claramunt RM, Roussel C, Alkorta I, Elguero J (2018). Indian J Heterocycl Chem 28:1–10
London F (1937). J Phys Radium 8:397–409
Ditchfield R (1974). Mol Phys 27:789–807
Gaussian 09 (2009) Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr., JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian, Inc., Wallingford CT
AMS S, RMS S, Jimeno ML, Blanco F, Alkorta I, Elguero J (2008). Magn Reson Chem 46:859–864
Blanco F, Alkorta I, Elguero J (2007). Magn Reson Chem 45:797–800
Groom CR, Bruno IJ, Lightfoot MP, Ward SC, The Cambridge Structural Database (2016) The Cambridge structural database. Acta Crystallogr Sect B 72:171–179. https://doi.org/10.1107/S2052520616003954
Joyce SA, Yates JR, Pickard CJ, Mauri F (2007). J Chem Phys 127:204107
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MJ, Refson K, Payne MC (2005). Z Kristallogr 220:567–570
Marín-Luna M, Claramunt RM, Nieto CI, Alkorta I, Elguero J, Reviriego F (2019). A theoretical NMR study of polymorphism in crystal structures of azoles and benzazoles. Magn Reson Chem. https://doi.org/10.1002/mrc.4824
Butler RN, Stephens JC, Burke LA (2003). Chem Commun:1016–1017
Butler RN, Hanniffy JM, Stephens JC, Burke LA (2008). J Organomet Chem 73:1354–1364
Huang H, Zhong J, Ma L, Lv L, Francisco JS, Zeng XC (2019). J Am Chem Soc 141:2984–2989. https://doi.org/10.1021/jacs.8b11335
Carpino LA, Padykula RE, Barr DE, Hall FH, Krause JG, Dufresne RF, Thoman CJ (1988). J Organomet Chem 53:2565–2572
Hansch C, Leo A (1995) Exploring QSAR: Fundamentals and Applications in Chemistry and Biology. American Chemical Society, Whashington, DC
Alkorta I, Elguero J (2018). Chem Phys Lett 691:33–36
Elguero J, Marzin C, Tizané D (1969). Org Magn Reson 1:249–275
Hung TQ, Dang TT, Janke J, Villinger A, Langer P (2015). Org Biomol Chem (13):1375–1386
Funding
This work was carried out with financial support from the Spanish Ministerio de Ciencia, Innovación y Universidades (Projects PGC2018-094644-B-C2 and RTI2018-097416-B-C21) and Dirección General de Investigación e Innovación de la Comunidad de Madrid (PS2018/EMT-4329 AIRTEC-CM). Thanks are also given to the CTI (CSIC) for their continued computational support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 523 kb)
Rights and permissions
About this article
Cite this article
Holzer, W., Castoldi, L., Kyselova, V. et al. Multinuclear NMR spectra and GIAO/DFT calculations of N-benzylazoles and N-benzylbenzazoles. Struct Chem 30, 1729–1735 (2019). https://doi.org/10.1007/s11224-019-01310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01310-3