Abstract
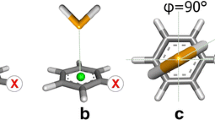
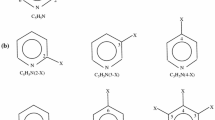
Contacts between aromatic rings and sulfur-containing amino acids are frequent in proteins. However, little is known about the nature of their interactions particularly if substituents are present on aromatic ring. In this paper, DFT quantum chemical calculations were used to study substituted benzenes in complex with hydrogen sulfide (H2S), methanethiol (CH3SH), and (Methylsulfanyl)methane (CH3SCH3). It was found that SH···π interaction is more stabilizing than the S···π interaction in the case of benzene, but this is changed with increasing electronegativity of the substituent on benzene ring. Although the change of energy of SH···π and S···π interaction follows the conventional model of substituent effect, where S···π interactions are maximized and SH···π interactions are diminished with electron-withdrawing substituent on benzene as a result of changes in the aryl π-system, it was found that it is mainly a consequence of direct electrostatic interaction between substituent and the sulfur-containing molecule. We also investigated the model system of Cys···Trp interaction, adjacent to a cluster of aromatic amino acids, in proteins, using explicit membrane molecular dynamics simulations results of D3 dopamine receptor crystal structure as starting point. It was found that fluorination in aromatic cluster enhances the Cys···Trp interaction. The effect is maximized when transferred through the rest of aromatic system suggesting possible explanation for frequent contacts between sulfur-containing and aromatic amino acids in proteins and their effects on protein folding and stabilization.
















Similar content being viewed by others
References
Morgan RS, Tatsch CE, Gushard RH, McAdon J, Warme PK (1978) Int J Pept Prot Res 11:209–217
Bodner BL, Jackman LM, Morgan RS (1980) Biochem Biophys Res Commun 94:807–813
Pal D, Chakrabarti P (2001) J Biomol Struct Dyn 19:115–128
Cordomi A, Gomez-Tamayo JC, Gigoux V, Fourmy D (2013) Trends Pharmacol Sci 34(6):320–331
Duan G, Smith VH Jr, Weaver DF (2001) Mol Phys 19:1689–1699
Meyer EA, Castellano RK, Diederich F (2003) Angew Chem Int Ed 42(11):1210–1250
Tatko CD, Waters ML (2004) Protein Sci 13(9):2515–2522
Tauer TP, Derrick ME, Sherrill CD (2005) J Phys Chem A 109:191–196
Yan S, Lee SJ, Kang S, Choi KH, Rhee SK, Lee JY (2007) Bull Korean Chem Soc 28(6):959–964
Ringer AL, Senenko A, Sherrill CD (2007) Protein Sci 16:2216–2223
Wheeler SE (2013) Acc Chem Res 46:1029–1038
Wheeler SE, Houk KN (2008) J Am Chem Soc 130:10854–10855
Valley CC, Cembran A, Perlmutter JD, Lewis AK, Labello NP, Gao J, Sachs JN (2012) J Biol Chem 287(42):34979–34991
Daeffler KNM, Laster HA, Dougherty DA (2012) J Am Chem Soc 134:14890–14896
Grimme S (2006) J Comput Chem 27:1787–1799
Schaefer A, Horn H, Ahlrichs R (1992) J Chem Phys 97:2571–2577
Schaefer A, Huber C, Ahlrichs R (1994) J Chem Phys 100:5829–5835
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian Inc, Wallingford CT
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K (2005) J Comput Chem 26:1781–1802
Chien EYT, Liu W, Zhao Q, Katritch V, Won Han G, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330(6007):1091–1095
Humphrey W, Dalke A, Schulten K (1996) J. Mol Graph 14:33–38
MacKerell AD Jr, Feig M, Brooks CL (2004) J Comput Chem 25:1400–1415
MacKerell AD Jr, Bashford D, Bellott M, Dunbrack RL Jr, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph- McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Wiorkiewicz-Kuczera J, Watanabe M, Yin D, Karplus M (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 102:3586–3616
Feller SE, Gawrisch G, MacKerell AD Jr (2002) J Am Chem Soc 124:318–326
Feller S, MacKerell AD Jr (2000) J Phys Chem B 104:7510–7515
PARADOX cluster at the Scientific Computing Laboratory of the Institute of Physics Belgrade, supported in part by the Serbian Ministry of Education and Science under project No. ON171017, and by the European Commission under FP7 projects HP-SEE, PRACE-1IP, PRACE-2IP, EGI-InSPIRE
Origin (OriginLab, Northampton, MA). http://www.originlab.com/index.aspx?go=Company&pid=1130
Accelrys Software Inc. (2007) Discovery Studio Viewer, Release 3.5. Accelrys Software Inc., San Diego. http://accelrys.com/products/discovery-studio/
Persistence of Vision Pty. Ltd. (2004) Persistence of Vision Raytracer (Version 3.6) [Computer software]. http://www.povray.org/documentation/view/3.6.0/203/. Accesed 2 Dec 2014
Adobe Photoshop 7 (2002) Adobe Systems Inc. http://www.adobe.com/products/photoshopfamily.html. Accesed 2 Dec 2014
Dennington Roy, Keith Todd, Millam John (2009) GaussView, version 5. Semichem Inc., Shawnee Mission, KS
Hansch C, Leo A (1979) Substituent constants for correlation analysis in chemistry and biology. Wiley-Interscience, New York
Acknowledgments
This work was supported by the Ministry of Education and Science of the Republic of Serbia (Grant No. OI172032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senćanski, M., Došen-Mićović, L., Šukalović, V. et al. Theoretical insight into sulfur–aromatic interactions with extension to D2 receptor activation mechanism. Struct Chem 26, 1139–1149 (2015). https://doi.org/10.1007/s11224-015-0574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0574-z