Abstract
Background
It is well known that the antipsychotic drugs impact the health-related quality of life (HRQoL) of the bipolar patients. The side effects had been assessed only from the doctors’ perspective and neglected the patients’ subjective feeling. The aim of the study is to validate the specific instrument “tolerability and quality of life” (TOOL) into Chinese to describe and grade the impact of antipsychotic drugs on HRQoL from patients’ view.
Methods
A psychometric study was conducted with euthymic bipolar disorder patients (N = 105) under antipsychotic treatment. The psychometric properties of the TOOL, including internal consistency, retest reliability, concurrent validity, content validity, discriminative validity, item analysis, confirmatory factor analysis and feasibility, were analyzed.
Results
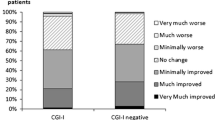
The internal consistency and intraclass correlation coefficient (ICC) were adequate (Cronbach’s alpha = 0.80 and ICC = 0.81). A confirmatory factor analysis (CFA) validated the one-factor model. Significant Spearman’s rank correlations between the TOOL and both Bref QoL.BD (Brief version of Quality of Life in Bipolar Disorder) (r = −0.33, P < 0.01) and UKU (Udvalg for Kliniske Undersogelser side effects scale) (r = 0.13, P < 0.05) were found.
Limitations
Small sample size and no specific self-report instrument in Chinese to evaluate the criterion validity.
Conclusions
TOOL appears to be a reliable and valid measure to assess the impact of adverse events of antipsychotic drugs on HRQoL from the patients’ perspective.
Similar content being viewed by others
References
Buckley, P. F. (2008). Update on the etiology and treatment of schizophrenia and bipolar disorder. CNS Spectrums, 13(Suppl 1), 3–10.
Edwards, S. J., & Smith, C. J. (2009). Tolerability of atypical antipsychotics in the treatment of adults with schizophrenia or bipolar disorder: A mixed treatment comparison of randomized controlled trials. Clinical Therapeutics, 31, 1345–1359.
Elmslie, J. L., Silverstone, J. J., Mann, J. I., Williams, S. M., & Romans, S. E. (2000). Prevalence of overweight and obesity in bipolar patients. Journal of Clinical Psychiatry, 61(3), 179–184.
Fagiolini, A., Chengappa, K. N., Soreca, I., & Chang, J. (2008). Bipolar disorder and the metabolic syndrome: Causal factors, psychiatric outcomes and economic burden. CNS Drugs, 22(8), 655–669.
Kolotkin, R. L., Corey, L., & Crosby, R. D. (2008). Impact of obesity on HRQoL in schizophrenia and bipolar disorder. Obesity, 16, 749–754.
Berk, M., & Berk, L. (2003). Mood stabilizers and treatment adherence in bipolar disorder: Addressing adverse events. Annals of Clinical Psychiatry, 15(3), 217–224.
Chue, P., & Kovacs, C. S. (2003). Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: Prevalence, monitoring and management. Bipolar Disorders, 5(Suppl 2), 62–79.
Johnson, F. R., Ozdemir, S., Manjunath, R., Hauber, A. B., Burch, S. P., & Thompson, T. R. (2007). Factors that affect adherence to bipolar disorder treatments: A stated-preference approach. Medical Care, 45(6), 545–552.
Sachs, G. S. (2003). Unmet clinical needs in bipolar disorder. Journal of Clinical Psychopharmacology, 23(Suppl 1), S2–S8.
Lingam, R., & Scott, J. (2002). Treatment non-adherence in affective disorders. Acta Psychiatrica Scand., 105(3), 164–172.
Endicott, J., Paulsson, B., Gustafsson, U., Schioler, H., & Hassan, M. (2008). Quetiapine monotherapy in the treatment of depressive episodes of bipolar I and II disorder: Improvements in quality of life and quality of sleep. Journal of Affective Disorders, 111(2–3), 306–319.
Yen, C. F., Cheng, C. P., Huang, C. F., Yen, J. Y., Ko, C. H., & Chen, C. S. (2008). Quality of life and its association with insight, adverse effects of medication and use of atypical antipsychotics in patients with bipolar disorder and schizophrenia in remission. Bipolar Disorders, 10(5), 617–624.
Awad, A. G., Hogan, T. P., Voruganti, L. N., & Heslegrave, R. J. (1995). Patients’ subjective experiences on antipsychotic medications: Implications for outcome and quality of life. International Clinical Psychopharmacology, 10, 123–132.
Ritsner, M., Modai, I., Endicott, J., Rivkin, O., Nechamkin, Y., Barak, P., et al. (2000). Differences in quality of life domains and psychopathologic and psychosocial factors in psychiatric patients. Journal of Clinical Psychiatry, 61(11), 880–889.
Reine, G., Lancon, C., Di, T. S., Sapin, C., & Auquier, P. (2003). Depression and subjective quality of life in chronic phase schizophrenic patients. Acta Psychiatrica Scandinavica, 108(4), 297–303.
Taksh, U. (2006). A critical review of rating scales in the assessment of movement disorders in schizophrenia. Current Drug Targets, 7(9), 1225–1229.
Lingjaerde, O., Ahlfors, U. G., Bech, P., Dencker, S. J., & Elgen, K. (1987). The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatrica Scandinavica, 334, 1–100.
Thornicroft, G., & Tansella, M. (2005). Growing recognition of the importance of service user involvement in mental health service planning and evaluation. Epidemiologia e Psichiatria Sociale, 14(1), 1–3.
Montejo, A. L. (2008). Prolactin awareness: An essential consideration for physical health in schizophrenia. European Neuropsychopharmacology, 18(Suppl 2), S108–S114.
Montejo, A. L., Correas-Lauffer, J., Mauriño, J., Villa, G., Rebollo, P., Díez, T., & Cordero, L. (2011). Estimation of a multiattribute utility function for the Spanish version of the TOOL questionnaire. Value in Health, 14(4), 564–570.
Lindstrom, E., Jonsson, L., & Berntsson, A. (2009). A patient perspective on side effects of antipsychotic therapy: The TOOL instrument. Value in Health, 12(7), A361.
Jonsson, L., Lang, A., & Lindstrom, E. (2009). TOOL: Multi-attribute utility function reflecting patient experience of side effects to antipsychotic therapy. Value in Health, 12(7), A361.
Montejo, A. L., Lauffer, J. C., Cuervo, J., Rebollo, P., Cordero, L., Diez, T., & Maurino, J. (2011). Validation of a specific measure to assess health related quality of life in patients with schizophrenia and bipolar disorder: The ‘Tolerability and quality of life’ (TOOL) questionnaire. Annals of General Psychiatry, 10(1), 6.
Maurino, J., Cordero, L., Montejo, A. L., Correas-Lauffer, J., Cuervo, J., Rebollo, P., et al. (2009). The Spanish version of the TOOL questionnaire: Validation of a specific measure to evaluate health related quality of life in patients with schizophrenia and bipolar disorder. Value in Health, 12(7), A362.
Maurino, J., Cordero, L., Montejo, A., Rebollo, P., & Cuervo, J. (2008). The Spanish version of the TOOL questionnaire: A useful measure for evaluating the HRQoL and utilities from schizophrenic and bipolar patients. Value in Health, 11(6), A593.
Gandek, B., & Ware, J. E. (1998). Methods for validating and norming translations of health status questionnaires: The IQOLA project approach. Journal of Clinical Epidemiology, 51(11), 953–959.
Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62.
Bech. (1979). The Bech-Rafaelsen Mania Scale and Hamilton Depression Scale. Acta Psychiatrica Scandinavica, 59, 420–429.
Zhang, Z. J. (2001). Hamilton Depression Scale. Chinese Journal of Behavioral Medical Science, 10, 134–135.
Cui, S. (1985). Bech-Rafaelsen Mania Rating Scale for the assessment of manic patients. Chinese Journal of Nervous and Mental Diseases, 18, 267–269.
Michalak, E. E., Murray, G., & CREST.BD. (2010). Development of the QoL.BD: A disorder specific scale to assess quality of life in bipolar disorder. Bipolar Disorders, 12(7), 727–740.
Xiao, L., Gao, Y. L., Zhang, L. L., Chen, P. Y., Sun, X. J., & Tang, S. Y. (2016). Validity and reliability of the Brief version of Quality of Life in Bipolar Disorder (Bref QoL.BD) among Chinese bipolar patients. Journal of Affective Disorders, 193, 66–72.
Polit, D. F., & Beck, C. T. (2006). The content validity index: Are you sure you know what’s being reported? critique and recommendations. Research in Nursing & Health, 29(5), 489–497. (Chinese journal).
Shi, J. C., Mo, X. K., & Sun, Z. Q. (2012). Content validity index in scale development. Journal of Central South University, 37(2), 152–155. (Chinese journal).
Joreskog, K. G., & Sorbom, D. (1996). LISREL 8: User’s reference guide. Chicago: Scientific Software International.
Kelloway, E. K. (1998). Using LISREL for structural equation modeling: A researcher’s guide. Thousand Oaks, CA: SAGE.
Kline, R. B. (1998). Principals and practice of structural equation modeling. New York, NY: Guilford.
Guan, M. (2016). Factor structure of the Chinese version of the geriatric anxiety inventory. Annals of General Psychiatry, 15, 4.
Bollen, K. A. (1989). Structural equation with latent variables. New York, NY: Wiley.
Bonnin, C. M., Sanchez-Moreno, J., Martinez-Aran, A., Sole, B., Reinares, M., Rosa, A. R., et al. (2012). Subthreshold symptoms in bipolar disorder: Impact on neurocognition, quality of life and disability. Journal of Affective Disorders, 136(3), 650–659.
Cochran, W. G. (1952). The χ2 test of goodness of fit. The Annals of Mathematical Statistics, 23(3), 315–345.
Michalak, E. E., Yatham, L. N., & Lam, R. W. (2005). Quality of life in bipolar disorder: A review of the literature. Health and Quality of Life Outcomes, 3, 72.
Morselli, P. L., Elgie, R., & Cesana, B. M. (2004). GAMIAN-Europe/BEAM survey II: Cross-national analysis of unemployment, family history, treatment satisfaction and impact of the bipolar disorder on life style. Bipolar Disorders, 6(6), 487–497.
Endicott, J., Rajagopalan, K., Minkwitz, M., & Macfadden, W. (2007). BOLDER Study Group. A randomized, double-blind, placebo-controlled study of quetiapine in the treatment of bipolar I and II depression: Improvements in quality of life. International Clinical Psychopharmacology, 22, 29–37.
Vornik, L. A., & Hirschfeld, R. M. (2005). Bipolar disorder: Quality of life and the impact of atypical antipsychotics. The American Journal of Managed Care, 11(Suppl. 9), 275–280.
Perkins, D. O., Gu, H., Weiden, P. J., McEvoy, J. P., Hamer, R. M., & Lieberman, J. A. (2008). Predictors of treatment discontinuation and medication non-adherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: A randomized, double-blind, flexible-dose, multicenter study. Journal of Clinical Psychiatry, 69(1), 106–113.
Briggs, A., Wild, D., Lees, M., Reaney, M., Dursun, S., Parry, D., & Mukherjee, J. (2008). Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: Direct utility elicitation. Health and Quality of Life Outcomes, 6, 105.
Hayhurst, H., Palmer, S., Abbott, R., Johnson, T., & Scott, J. (2006). Measuring health-related quality of life in bipolar disorder: Relationship of the EuroQol (EQ-5D) to condition-specific measures. Quality of Life Research, 15(7), 1271–1280.
Acknowledgments
We thank all the participants and their caregivers that consented to participate in the study. And we appreciate the approval and support of the psychiatric wards of the Second Xiangya Hospital.
Funding
This study was funded by the Central South University Education, Grants 2014zzts329.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests regarding the publication of this paper.
Ethical approval
The Ethics Committee of the Second Xiangya Hospital of Central South University approved this study in March 2014.
Appendix
Appendix
English version of TOOL questionnaire (Montejo et al. [20])
Likert scale: 1 = minimum to 4 = maximum impact | |
1. Mood | 2. Function capabilities |
① I do not feel anxious or depressed | ① I have no difficulties with my usual activities |
② I feel a little anxious or depressed | ② I have some difficulties with my usual activities |
③ I feel rather much anxious or depressed | ③ I have rather much difficulties with my usual activities |
④ I feel very anxious or depressed | ④ I have great difficulties with my usual activities |
3. Fatigue or weakness | 4. Body weight |
① I do not feel tired or weak | ① I am not overweight |
② I feel a little tired or weak | ② I am a little overweight |
③ I feel rather much tired or weak | ③ I am rather much overweight |
④ I feel very tired or weak | ④ I am very overweight |
5. Stiffness or tremor | 6. Physical restlessness |
① I have no problems with stiffness or tremor | ① I feel no bodily restlessness |
② I have some problems with stiffness or tremor | ② I feel some bodily restlessness |
③ I have rather much problems with stiffness or tremor | ③ I feel rather much bodily restlessness |
④ I have great problems with stiffness or tremor | ④ I feel very much bodily restlessness |
7. Sexual function | 8. Dizziness or nausea |
① I have normal sexual lust and ability | ① I have no problems with dizziness or nausea |
② I have somewhat reduced sexual lust and ability | ② I have some problems with dizziness or nausea |
③ I have very reduced sexual lust and ability | ③ I have rather much problems with dizziness or nausea |
④ I have no sexual lust and ability | ④ I have great problems with dizziness or nausea |
Chinese version of TOOL questionnaire
李克特等级评分,4级评分 | |
1. 心境状态 | 2. 功能状态 |
① 我没有感觉到焦虑或抑郁 | ① 我应对日常活动没有困难 |
② 我感觉到有一点焦虑或抑郁 | ② 我应对日常活动有一点困难 |
③ 我感觉到比较多的焦虑或抑郁 | ③ 我应对日常活动有比较多的困难 |
④ 我感觉到非常焦虑或抑郁 | ④ 我应对日常活动有非常多的困难 |
3. 疲乏感或虚弱感 | 4. 体重 |
① 我没有感觉疲劳或虚弱 | ① 我没有超重 |
② 我感觉有一点疲劳或虚弱 | ② 我有一点超重 |
③ 我感觉比较疲劳或虚弱 | ③ 我超重比较多 |
④ 我感觉非常疲劳或虚弱 | ④ 我超重非常多 |
5. 僵硬或震颤 | 6. 身体坐立不安 |
① 我没有感到僵硬或震颤 | ① 我没有感到身体坐立不安 |
② 我感到有一点僵硬或震颤 | ② 我感到身体有一点坐立不安 |
③ 我感到比较僵硬或震颤 | ③ 我感到身体比较坐立不安 |
④ 我感到非常僵硬或震颤 | ④ 我感到身体非常坐立不安 |
7. 性功能 | 8. 眩晕或恶心 |
① 我的性欲望及性能力正常 | ① 我没有感到眩晕或恶心 |
② 我的性欲望及性能力有一点下降 | ② 我感觉有一点眩晕或恶心 |
③ 我的性欲望及性能力下降比较多 | ③ 我感觉比较眩晕或恶心 |
④ 我的性欲望及性能力消失 | ④ 我感觉非常眩晕或恶心 |
Rights and permissions
About this article
Cite this article
Xiao, L., Gao, Y., Zhang, L. et al. Adaptation and validation of the “tolerability and quality of life” (TOOL) questionnaire in Chinese bipolar patients. Qual Life Res 25, 2825–2832 (2016). https://doi.org/10.1007/s11136-016-1319-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1319-1