Abstract
Purpose
Hereditary angioedema (HAE) is a rare disorder characterized by highly variable, acute attacks of swelling at various anatomical locations. Clinical measures do not adequately assess the diversity of symptoms characteristic of an attack. Two disease-specific, patient-reported outcome measures were developed to comprehensively capture symptom severity and change: the Treatment Outcome Score (TOS) and the Mean Symptom Complex Severity (MSCS) score.
Methods
This study comprised a secondary analysis of pooled data from a randomized controlled trial to evaluate the psychometric properties, including reliability and validity, and minimally important difference (MID) of the TOS and MSCS score.
Results
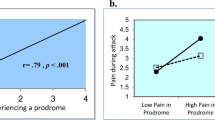
HAE patients (n = 73) had a mean age of 33 years, and 60% were female. Test–retest evaluation demonstrated moderate to substantial agreement (ICCs = 0.53 for TOS; 0.62 for MSCS score). The TOS and change in MSCS score were moderately to highly correlated with a Global Improvement Measure at 4 h (TOS: r = 0.90; MSCS: r = −0.59). Anchor- and distribution-based analyses suggested that conservative estimates for MID are 30 points for TOS and −0.30 points for 4-h change in MSCS score.
Conclusions
The psychometric tests performed here provide evidence of the reliability and validity of the TOS and MSCS for evaluating symptom severity and change in HAE patients. The TOS and MSCS score provide an example of measurement methodology that may be used to precisely capture symptom severity and change in a disease characterized by acute attacks.


Similar content being viewed by others
References
Davis, A. E., 3rd. (2004). Biological effects of C1 inhibitor. Drug News & Perspectives, 17(7), 439–446.
Huang, Y. T., Lin, Y. Z., Wu, H. L., Chiu, T. F., Lee, K. M., Tsai, H. Y., et al. (2005). Hereditary angioedema: A family study. Asian Pacific Journal of Allergy and Immunology, 23(4), 227–233.
Tosi, M. (1998). Molecular genetics of C1 inhibitor. Immunobiology, 199(2), 358–365.
Cicardi, M., & Agostoni, A. (1996). Hereditary angioedema. New England Journal of Medicine, 334(25), 1666–1667.
Bracho, F. A. (2005). Hereditary angioedema. Current Opinion in Hematology, 12(6), 493–498.
Bork, K., Meng, G., Staubach, P., & Hardt, J. (2006). Hereditary angioedema: New findings concerning symptoms, affected organs, and course. American Journal of Medicine, 119(3), 267–274.
Bork, K., Hardt, J., Schicketanz, K. H., & Ressel, N. (2003). Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Archives of Internal Medicine, 163(10), 1229–1235.
Gompels, M. M., Lock, R. J., Abinun, M., Bethune, C. A., Davies, G., Grattan, C., et al. (2005). C1 inhibitor deficiency: Consensus document. Clinical and Experimental Immunology, 139(3), 379–394.
Levy, J. H., & O’Donnell, P. S. (2006). The therapeutic potential of a kallikrein inhibitor for treating hereditary angioedema. Expert Opinion on Investigational Drugs, 15(9), 1077–1090.
Hays, R., & Revicki, D. A. (2005). Reliability and validity (including responsiveness). In R. Hays & D. A. Revicki (Eds.), Assessing quality of life in clinical trials (pp. 25–39). New York, NY: Oxford University Press.
Revicki, D. A., Osoba, D., Fairclough, D., Barofsky, I., Berzon, R., Leidy, N. K., et al. (2000). Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Quality of Life Research, 9(8), 887–900.
Leidy, N. K., Revicki, D. A., & Geneste, B. (1999). Recommendations for evaluating the validity of quality of life claims for labeling and promotion. Value Health, 2(2), 113–127.
Vernon, M. K., Rentz, A., White, M., & Schmalbach, T. (2007). Content validity and usability of the treatment outcome score and mean complex severity score: New electronic pros to assess symptom severity and improvement in hereditary angioedema. In 14th Annual Conference of the International Society for Quality of Life Research, Toronto, Ontario, Canada.
White, M., Vernon, M. K., Rentz, A., Beck, T. R., & Roberts, J. (2007). Development of a Patient-Reported Outcome (PRO) Measure to Assess Symptom Severity and Change in Hereditary Angioedema (HAE). The American College of Allergy, Asthma, & Immunology Annual Scientific Meeting, Dallas, TX.
Vernon, M. K., Rentz, A. M., White, M. V., & Grienenberger, A. (2008). Validation of a Patient-Reported Outcome Measure to Assess Response to Treatment in Hereditary Angioedema. American College of Asthma, Allergy, & Immunology, Seattle, WA.
Schneider, L., Lumry, W., Vegh, A., Williams, A. H., & Schmalbach, T. (2007). Critical role of kallikrein in hereditary angioedema pathogenesis: A clinical trial of ecallantide, a novel kallikrein inhibitor. The Journal of Allergy and Clinical Immunology, 120(2), 416–422.
Kunschak, M., Engl, W., Maritsch, F., Rosen, F. S., Eder, G., Zerlauth, G., et al. (1998). A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion, 38(6), 540–549.
Waytes, A. T., Rosen, F. S., & Frank, M. M. (1996). Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. New England Journal of Medicine, 334(25), 1630–1634.
Saengpanich, S., deTineo, M., Naclerio, R. M., & Baroody, F. M. (2003). Fluticasone nasal spray and the combination of loratadine and montelukast in seasonal allergic rhinitis. Archives of Otolaryngology: Head & Neck Surgery, 129(5), 557–562.
Simons, F. E., Prenner, B. M., & Finn, A., Jr. (2003). Efficacy and safety of desloratadine in the treatment of perennial allergic rhinitis. The Journal of Allergy and Clinical Immunology, 111(3), 617–622.
Small, C. B., Hernandez, J., Reyes, A., Schenkel, E., Damiano, A., Stryszak, P., et al. (2005). Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. The Journal of Allergy and Clinical Immunology, 116(6), 1275–1281.
Patrick, D. L., Burke, L. B., Powers, J. H., Scott, J. A., Rock, E. P., Dawisha, S., et al. (2007). Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health, 10(Suppl 2), S125–S137.
Deyo, R. A., Diehr, P., & Patrick, D. L. (1991). Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Controlled Clinical Trials, 12(4 Suppl), 142S–158S.
Streiner, D. L., & Norman, G. R. (2003). Health measurement scales. Oxford: Oxford University Press.
Scientific Advisory Committee of the Medical Outcomes Trust. (2002). Assessing health status and quality-of-life instruments: Attributes and review criteria. Quality of Life Research, 11(3), 193–205.
Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum.
Sloan, J. A., Symonds, T., Vargas-Chanes, D., & Friedly, B. (2003). Practical guidelines for assessing the clinical significance of heath related quality of life changes within clinical trials. Drug Information Journal, 37, 23–31.
Wyrwich, K. W., Nienaber, N. A., Tierney, W. M., & Wolinsky, F. D. (1999). Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Medical Care, 37(5), 469–478.
Wyrwich, K. W., Tierney, W. M., & Wolinsky, F. D. (1999). Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. Journal of Clinical Epidemiology, 52(9), 861–873.
Norman, G. R., Sloan, J. A., & Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Medical Care, 41(5), 582–592.
Acknowledgments
The authors thank Leslie E. Stolz, PhD (Dyax Corp.), and Mr. Fritz Hamme, BA (UBC) for assistance with the drafting of this manuscript.
Conflict of interest
Funding for this manuscript was provided by Dyax Corp. All data listings are available upon request. EDEMA3® and EDEMA4® are registered service marks of Dyax.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vernon, M.K., Rentz, A.M., Wyrwich, K.W. et al. Psychometric validation of two patient-reported outcome measures to assess symptom severity and changes in symptoms in hereditary angioedema. Qual Life Res 18, 929–939 (2009). https://doi.org/10.1007/s11136-009-9509-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-009-9509-8