Abstract
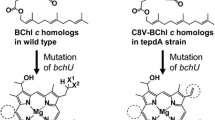
We recently constructed the mutant of the brown-colored green sulfur bacterium Chlorobaculum limnaeum lacking BciD which was responsible for formation of a formyl group at the 7-position in bacteriochlorophyll(BChl)-e biosynthesis. This mutant exclusively gave BChl-c, but not BChl-e, as the chlorosome pigments (Harada et al. in PLoS One 8(4):e60026, 2013). By the mutation, the homolog and epimer composition of the pigment was drastically altered. The methylation at the 82-position in the mutant cells proceeded to create BChl-c carrying large alkyl substituents at this position. Correspondingly, the content of BChls-c having the (S)-configuration at the chiral 31-position remarkably increased and accounted for 80.6 % of the total BChl-c. Based on the alteration of the pigment composition in the mutant cells, a new BChl-c bearing the bulkiest, triple 82-methylated neopentyl substituent at the 8-position ([N,E]BChl-c) was identified. The molecular structure of [N,E]BChl-c was fully determined by its NMR, mass, and circular dichroism spectra. The newly identified [N,E]BChl-c was epimerically pure at the chiral 31-position and its stereochemistry was determined to be an (S)-configuration by modified Mosher’s method. Further, the effects of the C82-methylation on the optical absorption properties of monomeric BChls-c were investigated. The Soret but not Qy absorption bands shifted to longer wavelengths by the extra methylation (at most 1.4 nm). The C82-methylation induced a slight but apparent effect on absorption properties of BChls-c in their monomeric states.






Similar content being viewed by others
Abbreviations
- APCI:
-
Atmospheric pressure chemical ionization
- BChl:
-
Bacteriochlorophyll
- BPhe:
-
Bacteriopheophytin
- Cba. :
-
Chlorobaculum
- CD:
-
Circular dichroism
- Chls:
-
Chlorophylls
- LCMS:
-
Liquid chromatography mass spectrometry
- MTPA:
-
α-Methoxy-α-(trifluoromethyl)phenylacetyl
- NOE:
-
Nuclear Overhauser effect
- PDA:
-
Photodiode array
- R[E,E]:
-
(31 R)-8-ethyl-12-ethyl
- R[E,M]:
-
(31 R)-8-ethyl-12-methyl
- R[I,E]:
-
(31 R)-8-isobutyl-12-ethyl
- R[P,E]:
-
(31 R)-8-propyl-12-ethyl
- ROESY:
-
Rotating frame Overhauser enhancement spectroscopy
- S[E,E]:
-
(31 S)-8-ethyl-12-ethyl
- S[I,E]:
-
(31 S)-8-isobutyl-12-ethyl
- S[N,E]:
-
(31 S)-8-neopentyl-12-ethyl
- S[P,E]:
-
(31 S)-8-propyl-12-ethyl
- THF:
-
Tetrahydrofuran
- TOF:
-
Time of flight
References
Balaban TS, Holzwarth AR, Schaffner K, Boender G-J, de Groot HJM (1995) CP-MAS 13C-NMR dipolar correlation spectroscopy of 13C-enriched chlorosomes and isolated bacteriochlorophyll c aggregates of Chlorobium tepidum: the self-organization of pigments is the main structural feature of chlorosomes. Biochemistry 34:15259–15266
Balaban TS, Tamiaki H, Holzwarth AR (2005) Chlorins programmed for self-assembly. In: Würthner F (ed) Supramolecular dye chemistry (Topics Curr Chem vol 258). Springer, Heidelberg, pp 1–38
Blankenship RE, Matsuura K (2003) Antenna complexes from green photosynthetic bacteria. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis (Adv Photosynth Respir vol 13), Chapter 6. Kluwer, Dordrecht, pp 195–217
Bobe FW, Pfennig N, Swanson KL, Smith KM (1990) Red shift of absorption maxima in Chlorobiineae through enzymatic methylation of their antenna bacteriochlorophylls. Biochemistry 29:4340–4348
Borrego CM, Garcia-Gil LJ (1995) Rearrangement of light harvesting bacteriochlorophyll homologues as a response of green sulfur bacteria to low light intensities. Photosynth Res 45:21–30
Borrego CM, Gerola PD, Miller M, Cox RP (1999) The molar extinction coefficient of bacteriochlorophyll e and the pigment stoichiometry in Chlorobium phaeobacteroides. Photosynth Res 60:257–264
Chew AGM, Frigaard N-U, Bryant DA (2004) Identification of BchV, a C-31 hydratase specific for hypermethylated bacteriochlorophyll c in Chlorobium tepidum. In: van der Est A, Bruce D (eds) Photosynthesis: fundamental aspects to global perspectives research. Allen Press, Lawrence, pp 875–877
Chew AGM, Frigaard N-U, Bryant DA (2007) Bacteriochlorophyllide c C-82 and C-121 methyltransferases are essential for adaptation to low light in Chlorobaculum tepidum. J Bacteriol 189:6176–6184
Frigaard N-U, Chew AGM, Maresca JA, Bryant DA (2006) Bacteriochlorophyll biosynthesis in green bacteria. In: Grimm B, Porra RJ, Rüdiger W, Scheer H (eds) Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications (Adv Photosynth Respir vol 25), Chapter 15. Springer, Dordrecht, pp 201–221
Glaeser J, Bañeras L, Rütters H, Overmann J (2002) Novel bacteriochlorophyll e structures and species-specific variability of pigment composition in green sulfur bacteria. Arch Microbiol 177:475–485
Harada J, Miyago S, Mizoguchi T, Azai C, Inoue K, Tamiaki H, Oh-oka H (2008) Accumulation of chlorophyllous pigments esterified with the geranylgeranyl group and photosynthetic competence in the CT2256-deleted mutant of the green sulfur bacterium Chlorobium tepidum. Photochem Photobiol Sci 7:1179–1187
Harada J, Mizoguchi T, Tsukatani Y, Noguchi M, Tamiaki H (2012) A seventh bacterial chlorophyll driving a large light-harvesting antenna. Sci Rep 2:671. doi:10.1038/srep00671
Harada J, Mizoguchi T, Satoh S, Tsukatani Y, Yokono M, Noguchi M, Tanaka A, Tamiaki H (2013) Specific gene bciD for C7-methyl oxidation in bacteriochlorophyll e biosynthesis of brown-colored green sulfur bacteria. PLoS One 8(4):e60026. doi:10.1371/journal.pone.0060026
Huster MS, Smith KM (1990) Biosynthetic studies of substituent homologation in bacteriochlorophylls c and d. Biochemistry 29:4348–4355
Ishii T, Kimura M, Yamamoto T, Kirihata M, Uehara K (2000) The effects of epimerization at the 31-position of bacteriochlorophylls c on their aggregation in chlorosomes of green sulfur bacteria. Control of the ratio of 31 epimers by light intensity. Photochem Photobiol 71:567–573
Kelly DR (1999) A new method for the determination of the absolute stereochemistry of aromatic and heteroaromatic alkanols using Mosher’s esters. Tetrahedron Asymmetry 10:2927–2934
Miyatake T, Tamiaki H (2005) Self-aggregates of bacteriochlorophylls-c, d and e in a light-harvesting antenna system of green photosynthetic bacteria: effect of stereochemistry at the chiral 3-(1-hydroxyethyl) group on the supramolecular arrangement of chlorophyllous pigments. J Photochem Photobiol C 6:89–107
Mizoguchi T, Hara K, Nagae H, Koyama Y (2000) Structural transformation among the aggregate forms of bacteriochlorophyll c as determined by electronic-absorption and NMR spectroscopies: dependence on the stereoisomeric configuration and on the bulkiness of the 8-C side chain. Photochem Photobiol 71:596–609
Mizoguchi T, Saga Y, Tamiaki H (2002) Isolation and structure determination of a complete set of bacteriochlorophyll-d homologs and epimers from a green sulfur bacterium Chlorobium vibrioforme and their aggregation properties in hydrophobic solvents. Photochem Photobiol Sci 1:780–787
Mizoguchi T, Oh-oka H, Tamiaki H (2005) Determination of stereochemistry of bacteriochlorophyll g F and 81-hydroxy-chlorophyll a F from Heliobacterium modesticaldum. Photochem Photobiol 81:666–673
Mizoguchi T, Harada J, Tamiaki H (2006) Structural determination of dihydro- and tetrahydrogeranylgeranyl groups at the 17-propionate of bacteriochlorophylls-a. FEBS Lett 580:6644–6648
Ohtani I, Kusumi T, Kashman Y, Kakisawa H (1991) High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J Am Chem Soc 113:4092–4096
Olson JM (1998) Chlorophyll organization and function in green photosynthetic bacteria. Photochem Photobiol 67:61–75
Orf GS, Blankenship RE (2013) Chlorosome antenna complexes from green photosynthetic bacteria. Photosynth Res 116:315–331
Otte SCM, van de Meent EJ, van Veelen PA, Pundsnes AS, Amesz J (1993) Identification of the major chlorosomal bacteriochlorophylls of the green sulfur bacteria Chlorobium vibrioforme and Chlorobium phaeovibrioides; their function in lateral energy transfer. Photosynth Res 35:15–169
Overmann J, Cypionka H, Pfennig N (1992) An extremely low-light-adapted phototrophic sulfur bacterium from the Black Sea. Limnol Oceanogr 37:150–155
Saga Y, Matsuura K, Tamiaki H (2001) Spectroscopic studies on self-aggregation of bacteriochlorophyll-e in nonpolar organic solvents: effects of stereoisomeric configuration at the 31-position and alkyl substituents at the 81-position. Photochem Photobiol 74:72–80
Scheer H (2006) An overview of chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. In: Grimm B, Porra RJ, Rüdiger W, Scheer H (eds) Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications (Adv Photosynth Respir vol 25), Chapter 1. Springer, Dordrecht, pp 1–26
Smith KM, Goff DA (1985) Bacteriochlorophylls-d from Chlorobium vibrioforme: chromatographic separations and structural assignments of the methyl bacteriopheophorbides. J Chem Soc Perkin Trans 1:1099–1113
Smith KM, Goff DA, Fajer J, Barkigia KM (1983a) Isolation and characterization of two novel bacteriochlorophylls d bearing neopentyl substituents. J Am Chem Soc 105:1674–1676
Smith KM, Craig W, Kehres LA, Pfennig N (1983b) Reversed-phase high-performance liquid chromatography and structural assignments of the bacteriochlorophylls-c. J Chromatogr 281:209–223
Steensgaard DB, Wackerbarth H, Hildebrandt P, Holzwarth AR (2000) Diastereoselective control of bacteriochlorophyll e aggregation. 31-S-BChl e is essential for the formation of chlorosome-like aggregates. J Phys Chem B 104:10379–10386
Tamiaki H (2005) Self-aggregates of natural and modified chlorophylls as photosynthetic light-harvesting antenna systems: substituent effect on the B-ring. Photochem Photobiol Sci 4:675–680
Tamiaki H, Kunieda M (2011) Photochemistry of chlorophylls and their synthetic analogs. In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science, vol 11, Chapter 51. World Scientific, Singapore, pp 223–289
Tamiaki H, Takeuchi S, Tsudzuki S, Miyatake T, Tanikaga R (1998) Self-aggregation of synthetic zinc chlorins with a chiral 1-hydroxyethyl group as a model for in vivo epimeric bacteriochlorophyll-c and d aggregates. Tetrahedron 54:6699–6718
Tamiaki H, Kitamoto H, Nishikawa A, Hibino T, Shibata R (2004) Determination of 31-stereochemistry in synthetic bacteriochlorophyll-d homologues and self-aggregation of their zinc complexes. Bioorg Med Chem 12:1657–1666
Tamiaki H, Shibata R, Mizoguchi T (2007) The 17-propionate function of (bacterio)chlorophylls: biological implication of their long esterifying chains in photosynthetic systems. Photochem Photobiol 83:152–162
Tamiaki H, Komada J, Kunieda M, Fukai K, Yoshitomi T, Harada J, Mizoguchi T (2011) In vitro synthesis and characterization of bacteriochlorophyll-f and its absence in bacteriochlorophyll-e producing organisms. Photosynth Res 107:133–138
Tsukatani Y, Harada J, Mizoguchi T, Tamiaki H (2013) Bacteriochlorophyll homolog compositions in the bchU mutants of green sulfur bacteria. Photochem Photobiol Sci 12:2195–2201
Vogl K, Tank M, Orf GS, Blankenship RE, Bryant DA (2012) Bacteriochlorophyll f: Properties of chlorosomes containing the “forbidden chlorophyll”. Front Microbiol 3. doi:10.3389/fmicb.2012.00298
Acknowledgments
We would like to thank Mr. D. Shoutsu, Dr. E. Matsuo, Ms. K. Arakawa, Mr. H. Terada, and Mr. Y. Yasui of Shimadzu Co., Ltd. for their experimental support of ultra-fast HPLC coupled with APCI-mass measurements. This work was partially supported by Grants-in-Aid for Scientific Research (A) (No. 22245030 to HT), (C) (No. 24550065 to TM) and for Young Scientists (B) (No. 24750169 to JH) as well as for Scientific Research on Innovative Areas (“Artificial Photosynthesis (AnApple)”, No. 24107002 to HT) from the Japan Society for the Promotion of Science (JSPS). YT is supported by a PRESTO (Precursory Research for Embryonic Science and Technology) fellowship from the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mizoguchi, T., Harada, J., Tsukatani, Y. et al. Isolation and characterization of a new bacteriochlorophyll-c bearing a neopentyl substituent at the 8-position from the bciD-deletion mutant of the brown-colored green sulfur bacterium Chlorobaculum limnaeum . Photosynth Res 121, 3–12 (2014). https://doi.org/10.1007/s11120-014-9977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-9977-8