Abstract
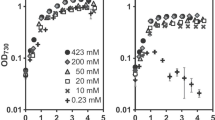
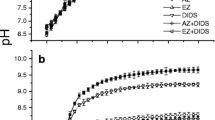
The extremophilic green microalga Chlamydomonas acidophila grows in very acidic waters (pH 2.3–3.4), where CO2 is the sole inorganic carbon source. Previous work has revealed that the species can accumulate inorganic carbon (Ci) and exhibits high affinity CO2 utilization under low-CO2 (air-equilibrium) conditions, similar to organisms with an active CO2 concentrating mechanism (CCM), whereas both processes are down-regulated under high CO2 (4.5 % CO2) conditions. Responses of this species to phosphorus (Pi)-limited conditions suggested a contrasting regulation of the CCM characteristics. Therefore, we measured external carbonic anhydrase (CAext) activities and protein expression (CAH1), the internal pH, Ci accumulation, and CO2-utilization in cells adapted to high or low CO2 under Pi-replete and Pi-limited conditions. Results reveal that C. acidophila expressed CAext activity and expressed a protein cross-reacting with CAH1 (the CAext from Chlamydomonas reinhardtii). Although the function of this CA remains unclear, CAext activity and high affinity CO2 utilization were the highest under low CO2 conditions. C. acidophila accumulated Ci and expressed the CAH1 protein under all conditions tested, and C. reinhardtii also contained substantial amounts of CAH1 protein under Pi-limitation. In conclusion, Ci utilization is optimized in C. acidophila under ecologically relevant conditions, which may enable optimal survival in its extreme Ci- and Pi-limited habitat. The exact physiological and biochemical acclimation remains to be further studied.





Similar content being viewed by others
References
Addanki A, Cahill FD, Sotos JF (1968) Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem 243:2337–2348
Badger MR, Kaplan A, Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii. Evidence for a carbon dioxide-concentrating mechanism. Plant Physiol 66:407–413
Balkos KD, Colman B (2007) Mechanism of CO2 acquisition in an acid-tolerant Chlamydomonas. Plant Cell Environ 30:745–752
Beardall J (1981) CO2 accumulation by Chlorella saccharophila (Chlorophyceae) at low external pH: evidence for active transport of inorganic carbon at the chloroplast envelope. J Phycol 17:371–373
Beardall J, Roberts S, Raven JA (2005) Regulation of inorganic carbon acquisition by phosphorus limitation in the green alga Chlorella emersonii. Can J Bot 83:859–864
Boron WF, Roos A (1976) Comparison of microelectrode, DMO, and methylamine methods for measuring intracellular pH. Am J Physiol 231:799–809
Buren S, Ortega-Villasante C, Blanco-Rivero A, Martinez-Bernardini A, Shutova T, Shevela D, Messinger J, Bako L, Villarejo A, Samuelsson G (2011) Importance of post-translational modifications for functionality of a chloroplast-localized carbonic anhydrase (CAH1) in Arabidopsis thaliana. Plos One 6(6):e21021
Coleman JR, Green LS, Berry JA, Togasaki RK, Grossman AR (1985) Adaptation of Chlamydomonas reinhardtii to air levels of CO2 and the induction of carbonic anhydrase activity. In: Berry JA, Lucas WJ (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. The American Society of Plant Physiologists, Rockwell, pp 339–359
Collins S, Bell G (2006) Evolution of natural algal populations at elevated CO2. Ecol Lett 9:129–135
Findenegg GR (1985) Ionic transport at the plasmalemma of Scenedesmus cells adapted to high and low CO2 levels. In: Berry JA, Lucas WJ (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. The American Society of Plant Physiologists, Rockwell, pp 155–168
Gehl KA, Cook CM, Colman B (1987) The effect of external pH on the apparent CO2 affinity of Chlorella saccharophila. J Exp Bot 38:1203–1210
Gehl KA, Colman B, Sposato LM (1990) Mechanism of inorganic carbon uptake in Chlorella saccharophila: the lack of involvement of carbonic anhydrase. J Exp Bot 41:1385–1391
Geib K, Golldack D, Gimmler H (1996) Is there a requirement for an external carbonic anhydrase in the extremely acid-resistant green alga Dunaliella acidophila? Eur J Phycol 31:273–284
Gerloff-Elias A, Spijkerman E, Pröschold T (2005) Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6). Plant Cell Environ 28:1218–1229
Gerloff-Elias A, Barua D, Mölich A, Spijkerman E (2006) Temperature- and pH-dependent accumulation of heat-shock proteins in the acidophilic green alga Chlamydomonas acidophila. FEMS Microbiol Ecol 56:345–354
Ghosh S, Gepstein S, Heikkila JJ, Dumbroff EB (1988) Use of a scanning densitometer or an ELISA plate reader for measurement of nanogram amounts of protein in crude extracts from biological tissues. Anal Biochem 169:227–233
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Hofstee BHJ (1952) On the evaluation of the constants Vm and Km in enzyme reactions. Science 116:329–331
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167:191–194
Kozlowska-Szerenos B, Bialuk I, Maleszewski S (2004) Enhancement of photosynthetic O2 evolution in Chlorella vulgaris under high light and increased CO2 concentration as a sign of acclimation to phosphate deficiency. Plant Physiol Bioch 42:403–409
Low-Decarie E, Jewell MD, Fussmann GF, Bell G (2013) Long-term culture at elevated atmospheric CO2 fails to evoke specific adaptation in seven freshwater phytoplankton species. Proc Royal Soc B: Biol Sci 280:20122598
Messerli MA, Amaral-Zettler LA, Zettler E, Jung SK, Smith PJS, Sogin ML (2005) Life at acidic pH imposes an increased energetic cost for a eukaryotic acidophile. J Exp Biol 208:2569–2579
Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109:133–149
Patel BN, Merrett MJ (1986) Regulation of carbonic anhydrase activity, inorganic carbon uptake and photosynthetic biomass yield in Chlamydomonas reinhardtii. Planta 169:81–86
Ratti S, Giordano M, Morse D (2007) CO2-concentrating mechanisms of the potentially toxic dinoflagellate Protoceratium reticulatum (Dinophyceae, Gonyaulacales). J Phycol 43:693–701
Satoh D, Hiraoka Y, Colman B, Matsuda Y (2001) Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Physiol 126:1459–1470
Spalding MH, Van K, Wang Y, Nakamura Y (2002) Acclimation of Chlamydomonas to changing carbon availability. Funct Plant Biol 29:221–230
Spijkerman E (2005) Inorganic carbon acquisition by Chlamydomonas acidophila across a pH range. Can J Bot 83:872–878
Spijkerman E (2008a) Phosphorus limitation of algae living in iron-rich, acidic lakes. Aquat Microb Ecol 53:201–210
Spijkerman E (2008b) What physiological acclimation supports increased growth at high CO2 conditions? Physiol Plant 133:41–48
Spijkerman E (2010) High photosynthetic rates under a colimitation for inorganic phosphorus and carbon dioxide. J Phycol 46:658–664
Spijkerman E (2011) The expression of a carbon concentrating mechanism in Chlamydomonas acidophila under variable phosphorus, iron, and CO2 concentrations. Photosynth Res 109:179–189
Spijkerman E, Bissinger V, Meister A, Gaedke U (2007) Low potassium and inorganic carbon concentrations influence a possible phosphorus limitation in Chlamydomonas acidophila (Chlorophyceae). Eur J Phycol 42:327–339
Spijkerman E, de Castro F, Gaedke U (2011) Independent colimitation for carbon dioxide and inorganic phosphorus. PLoS One 6:e28219
Spijkerman E, Wacker A, Weithoff G, Leya T (2012) Elemental and fatty acid composition of snow algae in Arctic habitats. Front Microbiol 3:380
Stojkovic S, Beardall J, Matear R (2013) CO2-concentrating mechanisms in three southern hemisphere strains of Emiliania huxleyi. J Phycol 49:670–679
Stumm W, Morgan JJ (1970) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley-Interscience, New York, p 583
Sültemeyer D, Fock HP, Canvin DT (1991) Active uptake of inorganic carbon by Chlamydomonas reinhardtii: evidence for simultaneous transport of HCO3 − and CO2 and characterization of active CO2 transport. Can J Bot 69:995–1002
Tittel J, Bissinger V, Gaedke U, Kamjunke N (2005) Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist 156:63–75
Tsuzuki M, Miyachi S, Berry JA (1985) Intracellular accumulation of inorganic carbon and its active species taken up by Chlorella vulgaris 11 h. In: Berry JA, Lucas WJ (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. The American Society of Plant Physiologists, Rockwell, pp 53–66
Van K, Spalding MH (1999) Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol 120:757–764
Villarejo A, Martinez F, Plumed MD, Ramazanov Z (1996) The induction of the CO2 concentrating mechanism in a starch-less mutant of Chlamydomonas reinhardtii. Physiol Plant 98:798–802
Villarejo A, Orus MI, Ramazanov Z, Martinez F (1998) A 38-kilodalton low-CO2-inducible polypeptide is associated with the pyrenoid in Chlorella vulgaris. Planta 206:416–425
Williams TG, Colman B (1995) Quantification of the contribution of CO2, HCO3 −, and external carbonic anhydrase to photosynthesis at low dissolved inorganic carbon in Chlorella saccarophila. Plant Physiol 107:245–251
Ynalvez RA, Xiao Y, Ward AS, Cunnusamy K, Moroney JV (2008) Identification and characterization of two closely related beta-carbonic anhydrases from Chlamydomonas reinhardtii. Physiol Plant 133:15–26
Young E, Beardall J, Giordano M (2001) Inorganic carbon acquisition by Dunaliella tertiolecta (Chlorophyta) involves external carbonic anhydrase and direct HCO3 − utilization insensitive to the anion exchange inhibitor DIDS. Eur J Phycol 36:81–88
Zenvirth D, Volokita M, Kaplan A (1985) Photosynthesis and inorganic carbon accumulation in the acidophilic alga Cyanidioschyzon merolae. Plant Physiol 77:237–239
Acknowledgments
ES acknowledges support from the German Science Foundation (SP695/4-2 and SP 695/5) and thanks Francesco Memmola, Anna Leetz, and Barbara Schmitz for practical assistance. Work in JB’s laboratory on inorganic carbon uptake, and its interactions with nutrient availability, was supported by the Australian Research Council. We are very grateful for the reviewers and editor’s comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spijkerman, E., Stojkovic, S. & Beardall, J. CO2 acquisition in Chlamydomonas acidophila is influenced mainly by CO2, not phosphorus, availability. Photosynth Res 121, 213–221 (2014). https://doi.org/10.1007/s11120-014-0016-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0016-6