Abstract
A novel Agrobacterium tumefaciens-mediated transient expression assay (AmTEA) was developed for young plants of different cereal species and the model dicot Arabidopsis thaliana. AmTEA was evaluated using five promoters (six constructs) and two reporter genes, gus and egfp. The constitutive 35S promoter and the promoter of the rice glutaredoxin gene showed gus and egfp expression in the cereals analyzed in the present study. A promoter for the DEAD-box RNA helicase family protein gene from Arabidopsis showed similar expression patterns of reporter genes in stable transgenic lines as well as in transient expression lines of Arabidopsis. Agrobacterium tumefaciens co-cultivation and plant incubation times were optimized using 35S and the rice expressed protein gene promoter (R2-273). The possibility of non-specific expression of the reporter genes was ruled out by using the antibiotic carbenicillin and the comparison of expression of the reporter genes driven by full-length and truncated R2-273 promoters. AmTEA considerably reduced time, space, labor, and cost requirements. Ease of use with stress treatments is another major advantage of this method. AmTEA can be automated and used for large-scale studies to decipher promoter and gene functions with the ultimate goal to enhance the performance of cereal crops against biotic and abiotic stresses.









Similar content being viewed by others
References
Amoah BK, Wu H, Sparks C, Jones HD (2001) Factors influencing Agrobacterium mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot 52:1135–1142
An Y-QC, Meagher RB (2010) Strong expression and conserved regulation of ACT2 in Arabidopsis thaliana and Physcomitrella patens. Plant Mol Biol Rep 28:481–490
Barton KA, Chilton MD (1983) Agrobacterium Ti plasmids as vectors for plant genetic engineering. Methods Enzymol 101:527–539
Bi C, Chen F, Jackson L, Gill BS, Li W (2011) Expression of lignin biosynthetic genes in wheat during development and upon infection by fungal pathogens. Plant Mol Biol Rep 29:149–161
da Silva JAT, Fukai S (2001) The impact of carbenicillin, cefotaxime and vancomycin on chrysanthemum and tobacco TCL morphogenesis and Agrobacterium growth. J Appl Hort 3:3–12
Chlan CA, Lin JM, Cary JW, Cleveland TE (1995) A procedure for biolistic transformation and regeneration of transgenic cotton from meristematic tissue. Plant Mol Biol Rep 13:31–37
Diao G, Wang Y, Wang C, Yang C (2011) Cloning and functional characterization of a novel glutathione S-transferase gene from Limonium bicolor. Plant Mol Biol Rep 29:77–87
Eckardt NA (2007) Oxidation pathways and plant development: crosstalk between thioredoxin and glutaredoxin pathways. Plant Cell 19:1719–1721
Fraley RT, Rogers SG, Horsch RB et al (1983) Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A 80:4803–4807
Fromm M, Taylor LP, Walbot V (1985) Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A 82:5824–5828
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Karimi M, Inze D, Depicker A (2002) Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Kato H, Xie G, Sato Y, Imai R (2010) Isolation of anther-specific gene promoters suitable for transgene expression in rice. Plant Mol Biol Rep 28:381–387
Li L, Qu R, Kochko AD, Fauquet C, Beachy RN (1993) An improved rice transformation system using the biolistic method. Plant Cell Rep 12:250–255
Li J-F, Park E, von Arnim AG, Nebenführ A (2009) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5:6
Liu Y-Z, Dong T, Lei Y, Deng X-X, Gu Q-Q (2011) Isolation of a polygalacturonase gene from Citrus sinensis fruit and its expression relative to fruit mastication trait, fruit development, and calcium or boron treatments. Plant Mol Biol Rep 29:51–59
Meng X, Li F, Liu C, Zhang C, Wu Z, Chen Y (2010) Isolation and characterization of an ERF transcription factor gene from cotton (Gossypium barbadense L.). Plant Mol Biol Rep 28:176–183
Qi J, Yu S, Zhang F, Shen X, Zhao X, Yu Y, Zhang D (2010) Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol Biol Rep 28:597–604
Rossi L, Escudero J, Hohn B, Tinland B (1993) Efficient assay for T-DNA dependent transient gene expression. Plant Mol Biol Rep 11:220–229
Saha D, Kumar V, Bhat SR, Srinivasan R (2011) Characterization of upstream sequences of the LOJ gene leads to identification of a novel enhancer element conferring lateral organ junction-specific expression in Arabidopsis thaliana. Plant Mol Biol Rep 29:265–277
Singer SD, Hily J-M, Liu Z (2010) A 1-kb bacteriophage lambda fragment functions as an insulator to effectively block enhancer–promoter interactions in Arabidopsis thaliana. Plant Mol Biol Rep 28:69–76
Uze M, Wunn J, Puonti-Kaerlas J, Potrykus I, Sautter C (1997) Plasmolysis of precultured immature embryos improves Agrobacterium mediated gene transfer to rice (Oryza sativa L.). Plant Science 130:87–95
Wang X, Dong J, Liu Y, Gao H (2010) A novel dehydration-responsive element-binding protein from Caragana korshinskii is involved in the response to multiple abiotic stresses and enhances stress tolerance in transgenic tobacco. Plant Mol Biol Rep 28:664–675
Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3:259–273
Acknowledgment
We thank USDA-GRIN for providing the seeds of rice, barley, maize, Sorghum, rye, and oats. This work was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2007-35301-18036. We thank the Michigan Technological University’s Biotech Research Centre (BRC) for their continuous support and funding. We personally thank Lorie Bernhardt (Dale Bumpers National Rice Research Center) for providing bulk quantities of rice seeds. We thank Dr. Chandrashekhar P. Joshi (Michigan Technological University) for the plasmid pBI121 and Dr. Victor Busov for Agrobacterium (GV3101). We also thank the Institute of Plant Systems Biology, Ghent University, Belgium, for pBGWFS7 and CAMBIA, Australia, for pCAMBIA2201.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
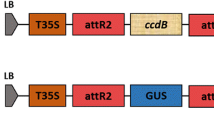
Figure S1
Organization of the vector pBGWFS7 (http://gateway.psb.ugent.be/vector/show/pBGWFS7/search/index). (PPT 211 kb)
Figure S2
Flow chart enumerating important steps in AmTEA. (PPT 58 kb)
Figure S3
Evaluation of wounding and non-wounding AmTEA with R2-273 promoter using rice plants. (PPT 190 kb)
Rights and permissions
About this article
Cite this article
Dhadi, S.R., Deshpande, A. & Ramakrishna, W. A Novel Non-wounding Transient Expression Assay for Cereals Mediated by Agrobacterium tumefaciens . Plant Mol Biol Rep 30, 36–45 (2012). https://doi.org/10.1007/s11105-011-0314-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-011-0314-5