Abstract
Purpose
This study investigated the effects of the physicochemical properties of antibiotics on the morphology, loading efficiency, size, release kinetics, and antibiotic efficacy of loaded poly(DL-lactic-co-glycolic acid) (PLGA) microparticles (MPs) at different loading percentages.
Methods
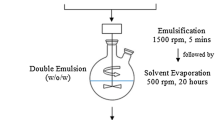
Cefazolin, ciprofloxacin, clindamycin, colistin, doxycycline, and vancomycin were loaded at 10 and 20 wt% into PLGA MPs using a water-in-oil-in water double emulsion fabrication protocol. Microparticle morphology, size, loading efficiency, release kinetics, and antibiotic efficacy were assessed.
Results
The results from this study demonstrate that the chemical nature of loaded antibiotics, especially charge and molecular weight, influence the incorporation into and release of antibiotics from PLGA MPs. Drugs with molecular weights less than 600 Da displayed biphasic release while those with molecular weights greater than 1,000 Da displayed triphasic release kinetics. Large molecular weight drugs also had a longer delay before release than smaller molecular weight drugs. The negatively charged antibiotic cefazolin had lower loading efficiency than positively charged antibiotics. Microparticle size appeared to be mainly controlled by fabrication parameters, and partition and solubility coefficients did not appear to have an obvious effect on loading efficiency or release. Released antibiotics maintained their efficacy against susceptible strains over the duration of release. Duration of release varied between 17 and 49 days based on the type of antibiotic loaded.
Conclusions
The data from this study indicate that the chemical nature of antibiotics affects properties of antibiotic-loaded PLGA MPs and allows for general prediction of loading and release kinetics.


Similar content being viewed by others
Abbreviations
- MIC:
-
Minimum inhibitory concentration
- MP:
-
Microparticle
- PBS:
-
Phosphate buffered saline
- PLGA:
-
Poly(DL-lactic-co-glycolic acid)
- PMMA:
-
Poly(methylmethacrylate)
- PVA:
-
Poly(vinyl alcohol)
References
Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. Staphylococcus aureus induces expression of receptor activator of NF- B ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun. 2008;76:5120–6.
Tiemann AH, Hofmann GO. Principles of the therapy of bone infections in adult extremities. Strat Traum Limb Recon. 2009;4:57–64.
Kent ME, Rapp RP, Smith KM. Antibiotic beads and osteomyelitis: here today, what’s coming tomorrow? Orthopedics. 2006;29:599–603.
Paladino JA, Poretz D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis. 2010;51 Suppl 2:S198–208.
Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–95.
Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5:160–70.
Anagnostakos K, Hitzler P, Pape D, Kohn D, Kelm J. Persistence of bacterial growth on antibiotic-loaded beads: is it actually a problem? Acta Orthop. 2008;79:302–7.
Shah SR, Tatara AM, D’Souza RN, Mikos AG, Kasper FK. Evolving strategies for preventing biofilm on implantable materials. Mater Today. 2013;16:177–82.
Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma. 1995;9:401–6.
Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Delivery Rev. 1997;28:5–24.
Shi M, Kretlow JD, Nguyen A, Young S, Baggett LS, Wong ME, et al. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials. 2010;31:4146–56.
Ramchandani M, Robinson D. In vitro and in vivo release of ciprofloxacin from PLGA 50:50 implants. J Controlled Release. 1998;54:167–75.
Özalp Y, Özdemir N, Kocagöz S, Hasirci V. Controlled release of vancomycin from biodegradable microcapsules. J Microencapsul. 2001;18:89–110.
Pillai RR, Somayaji SN, Rabinovich M, Hudson MC, Gonsalves KE. Nafcillin-loaded PLGA nanoparticles for treatment of osteomyelitis. Biomed Mater. 2008;3:034114–4.
Spicer PP, Shah SR, Henslee AM, Watson BM, Kinard LA, Kretlow JD, et al. Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model. Acta Biomater. 2013;11:1–31.
Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–76.
Calhoun J, Manring MM, Shirtliff M. Osteomyelitis of the long bones. Seminars in Plastic Surgery. 2009;23:059–72.
Mao S, Xu J, Cai C, Germershaus O, Schaper A, Kissel TT. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334:137–48.
Ghaderi R, Sturesson C, Carlfors J. Effect of preparative parameters on the characteristics of poly d, l-lactide-co-glycolide) microspheres made by the double emulsion method. Int J Pharm. 1996;141:205–16.
Wishart DS, Knox C, Guo AC, Cheng D. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–6.
Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, et al. Virtual computational chemistry laboratory–design and description. J Comput Aided Mol Des. 2005;19:453–63.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing [Internet]. Vienna, Austria. Available from: http://www.R-project.org/
Wahlig H, Dingeldein E, Buchholz HW, Buchholz M, Bachmann F. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg Br. 1984;66:175–9.
Chaisri W, Ghassemi AH, Hennink WE, Okonogi S. Enhanced gentamicin loading and release of PLGA and PLHMGA microspheres by varying the formulation parameters. Colloids and Surfaces B: Biointerfaces. 2011;84:508–14.
Pandey JD, Shukla A, Misra K, Rai RD. Ultrasonic, volumetric, and viscometric studies of tetracycline and its allied compound. J Chem Eng Data. 1989;34:29–31.
Weber A, Morlin G, Cohen M, Williams-Warren J, Ramsey B, Smith A. Effect of nebulizer type and antibiotic concentration on device performance. Pediatr Pulmonol. 1997;23:249–60.
Sherman P. The influence of internal phase viscosity on the viscosity of concentrated water-in-oil emulsions. Kolloid-Zeitschrift. 1955;141:6–11.
Mundargi RC, Srirangarajan S, Agnihotri SA, Patil SA, Ravindra S, Setty SB, et al. Development and evaluation of novel biodegradable microspheres based on poly(d, l-lactide-co-glycolide) and poly(@e-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: in vitro and in vivo studies. J Controlled Release. 2007;119:59–68.
Singh M, Briones M, Ott G, O’Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. PNAS. 2000;97:811–6.
Chun KW, Yoo HS, Yoon JJ, Park TG. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: effect of surface modification on cell attachment and function. Biotechnol Prog. 2004;20:1797–801.
Prior S, Gamazo C, Irache JM, Merkle HP, Gander B. Gentamicin encapsulation in PLA/PLGA microspheres in view of treatingBrucellainfections. Int J Pharm. 2000;196:115–25.
Vijan LE. The interaction of vancomycin with DNA. Rev Roum Chem. 2009;54:807–13.
Giovagnoli S, Tsai T, DeLuca PP. Formulation and release behavior of doxycycline–alginate hydrogel microparticles embedded into pluronic F127 thermogels as a potential new vehicle for doxycycline intradermal sustained delivery. AAPS PharmSciTech. 2010;11:212–20.
Falagas ME, Fragoulis KN, Karydis I. A comparative study on the cost of new antibiotics and drugs of other therapeutic categories. PLoS ONE. 2006;1:e11.
Ravi S, Peh KK, Darwis Y, Murthy BK, Singh TRR, Mallikarjun C. Development and characterization of polymeric microspheres for controlled release protein loaded drug delivery system. Indian J Pharm Sci. 2008;70:303–9.
Chaisri W, Hennink WE, Okonogi S. Preparation and characterization of cephalexin loaded PLGA microspheres. Curr Drug Delivery. 2009;6:69–75.
Frank A, Rath SK, Venkatraman SS. Controlled release from bioerodible polymers: effect of drug type and polymer composition. J Controlled Release. 2005;102:333–44.
Siegel SJ, Kahn JB, Metzger K, Winey KI, Werner K, Dan N. Effect of drug type on the degradation rate of PLGA matrices. Eur J Pharm Biopharm. 2006;64:287–93.
Maulding HV, Tice TR, Cowsar DR, Fong JW, Pearson JE, Nazareno JP. Biodegradable microcapsules: acceleration of polymeric excipient hydrolytic rate by incorporation of a basic medicament. J Controlled Release. 1986;3:103–17.
Lu L, Garcia CA, Mikos AG. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J Biomed Mater Res. 1999;46:236–44.
Sanders LM, McRae GI, Vitale KM, Kell BA. Controlled delivery of an LHRH analogue from biodegradable injectable microspheres. J Controlled Release. 1985;2:187–95.
Honnorat-Benabbou VC, Lebugle AA, Sallek B, Duffaut-Lagarrigue D. Stability study of tetracyclines with respect to their use in slow release systems. J Mater Sci: Mater Med. 2001;12:107–10.
Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Delivery Rev. 2001;47:229–50.
Donnelly RF. Stability of pantoprazole sodium in glass vials, polyvinyl chloride minibags, and polypropylene syringes. Can J Hosp Pharm. 2011;64:252–6.
Orwa JA, Govaerts C, Gevers K, Roets E, Van Schepdael A, Hoogmartens J. Study of the stability of polymyxins B 1, E 1 and E 2 in aqueous solution using liquid chromatography and mass spectrometry. J Pharm Biom Anal. 2002;29:203–12.
Raverdy V, Ampe E, Hecq JD, Tulkens PM. Stability and compatibility of vancomycin for administration by continuous infusion. J Antimicrob Chemother. 2013;68:1179–82.
Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Controlled Release. 2010;145:221–30.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-14-2-0004. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. SRS and PPS would like to acknowledge the Baylor College of Medicine Medical Scientist Training Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Correlations between loading efficiency (%) and charge (a,b), molecular weight (MW; c,d), logP (e,f), and logS (g,h) for 10 wt% loaded PLGA MPs (top row) and 20 wt% loaded PLGA MPs (bottom row). Charge has a positive correlation with loading efficiency for 10 wt% and 20 wt% loaded MPs. MW, logP, and logS have weak to very weak correlation with loading efficiency. (GIF 36 kb)
Figure S2
Correlations between release rate (%/day) during the major phase of release and charge (a,b), molecular weight (MW; c,d), logP (e,f), and logS (g,h) for 10 wt% loaded PLGA MPs (top row) and 20 wt% loaded PLGA MPs (bottom row). None of the factors investigated in this study correlate to the release rate. (GIF 34 kb)
Figure S3
Correlations between length of lag phase (days) and charge (a,b), molecular weight (MW; c,d), logP (e,f), and logS (g,h) for 10 wt% loaded PLGA MPs (top row) and 20 wt% loaded PLGA MPs (bottom row). MW has a very strong positive correlation with the length of the lag phase and a negative correlation with solubility. Charge and logP do not have strong correlations to length of lag phase. (GIF 33 kb)
Table SI
(DOCX 32 kb)
Table SII
(DOCX 62.4 kb)
Rights and permissions
About this article
Cite this article
Shah, S.R., Henslee, A.M., Spicer, P.P. et al. Effects of Antibiotic Physicochemical Properties on Their Release Kinetics from Biodegradable Polymer Microparticles. Pharm Res 31, 3379–3389 (2014). https://doi.org/10.1007/s11095-014-1427-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1427-y