ABSTRACT
Purpose
To study the potential impact of the degradation of Polysorbates (PS) 20 and 80 on the stability of therapeutic proteins in parenteral formulations.
Method
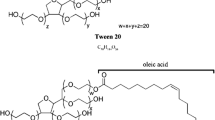
First, degradation products of PS20 and 80 were identified. Subsequently, the effect of degraded polysorbate on physical characteristics and long-term stability of protein formulations was assessed. Further, the impact of polysorbate degradation on protein stability was evaluated via shaking stress studies on formulations spiked with artificially degraded polysorbate or degradants like fatty acids. Additionally, aged formulations with reduced polysorbate content were shaken.
Results
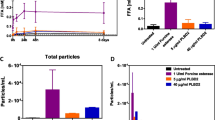
The degradation of polysorbate leads to a buildup of various molecules, some of which are poorly soluble, including fatty acids and polyoxyethylene (POE) esters of fatty acids. Spiking studies showed that the insoluble degradants could potentially impact protein stability and that the presence of sufficient intact polysorbate was crucial to prevent this. End-of-shelf-life shaking of protein formulations showed that the stability of various monoclonal antibodies was, however, not affected.
Conclusions
Although some degradants can potentially influence the stability of the protein (as discerned from spiking studies), degradation of polysorbates did not impact the stability of the different proteins tested in pharmaceutically relevant temperature and storage conditions.













Similar content being viewed by others
REFERENCES
Hillgren A, Lindgren J, Alden M. Protection mechanism of Tween 80 during freeze-thawing of a model protein, LDH. Int J Pharm. 2002;237:57–69.
Kiese S, Pappenberger A, Friess W, Mahler HC. Shaken, not stirred: mechanical stress testing of an IgG1 antibody. J Pharm Sci. 2008;97:4347–66.
Jones LS, Bam NB, Randolph TW. Surfactant-stabilized protein formulations: a review of protein-surfactant interactions and novel analytical methodologies. In: Shahrokh Z, Cleland JL, Shire SJ, editors. Therapeutic proteins and peptide formulation and delivery. Washington: American Chemical Society; 1997. p. 206–22.
Kreilgaard L, Jones LS, Randolph TW, Frokjaer S, Flink JM, Manning MC, et al. Effect of Tween 20 on freeze-thawing- and agitation-induced aggregation of recombinant human factor XIII. J Pharm Sci. 1998;87:1597–603.
Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289:1–30.
Mahler HC, Friess W, Grauschopf U, Kiese S. Protein aggregation: pathways, induction factors and analysis. J Pharm Sci. 2009;98:2909–34.
Mahler H-C, Mueller R, Friess W, Delille A, Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur J Pharm Biopharm. 2005;59:407–17.
Maaand Y-F, Hsu CC. Protein denaturation by combined effect of shear and air-liquid interface. Biotechnol Bioeng. 1997;54:503–12.
Cromwell MEM, Hilario E, Jacobson F. Protein aggregation and bioprocessing. Aaps J. 2006;8:E572–9.
Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW. Rational design of stable lyophilized protein formulations: theory and practice. Pharm Biotechnol. 2002;13:109–33.
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14:969–75.
Ayorinde FO, Gelain SV, Johnson Jr JH, Wan LW. Analysis of some commercial polysorbate formulations using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:2116–24.
Brandner JD. The composition of NF-defined emulsifiers: sorbitan monolaurate, monopalmitate, monostearate, monooleate, polysorbate 20, polysorbate 40, polysorbate 60, and polysorbate 80. Drug Dev Ind Pharm. 1998;24:1049–54.
Frison-Norrieand S, Sporns P. Investigating the molecular heterogeneity of polysorbate emulsifiers by MALDI-TOF MS. J Agric Food Chem. 2001;49:3335–40.
Wasylaschuk WR, Harmon PA, Wagner G, Harman AB, Templeton AC, Xu H, et al. Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci. 2007;96:106–16.
Ha E, Wang W, Wang YJ. Peroxide formation in polysorbate 80 and protein stability. J Pharm Sci. 2002;91:2252–64.
Harmon PA, Kosuda K, Nelson E, Mowery M, Reed RA. A novel peroxy radical based oxidative stressing system for ranking the oxidizability of drug substances. J Pharm Sci. 2006;95:2014–28.
Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 2008;97:2924–35.
Donbrow M, Azaz E, Pillersdorf A. Autoxidation of polysorbates. J Pharm Sci. 1978;67:1676–81.
Donbrow M, Hamburger R, Azaz E. Surface tension and cloud point changes of polyoxyethylenic nonionic surfactants during autoxidation. J Pharm Pharmacol. 1975;27:160–6.
Donbrow M, Hamburger R, Azaz E, Pillersdorf A. Development of acidity in nonionic surfactants: formic and acetic acid. Analyst (London). 1978;103:400–2.
Kishore RSK, Pappenberger A, Dauphin IB, Ross A, Buergi B, Staempfli A, Mahler HC. Degradation of polysorbates 20 and 80: Studies on thermal auto-oxidation in bulk and hydrolysis in formulations. J Pharm Sci. 2011;100:721–31.
Mahler H-C, Senner F, Maeder K, Mueller R. Surface activity of a monoclonal antibody. J Pharm Sci. 2009;98:4525–33.
Mahler HC, Huber F, Kishore RSK, Reindl J, Rückert P, Müller R. Adsorption behavior of a surfactant and a monoclonal antibody to sterilizing-grade filters. J Pharm Sci. 2010;99:2620–7.
Kiese S. Protein aggregation: induction, analytical methods and inhibition in biopharmaceutical formulations. Faculty of Chemistry und Pharmacy Vol. Doctorate, Ludwig-Maximillians-Universität München Munich, Germany, 2009, p. 298.
Carey FA, Sundberg RJ. Advanced organic chemistry, Springer Verlag, 2007.
Bates TR, Nightingale CH, Dixon E. Kinetics of hydrolysis of poly(oxyethylene) (20) sorbitan fatty acid ester surfactants. J Pharm Pharmacol. 1973;25:470–7.
Decker C, Marchal J. Autoxydation radio-induite du poly (oxyéthylène) en solution aqueuse, 7. Cinétique de la consommation d’oxygène. Die Makromolekulare Chemie. 1974;175:3531–40.
Dulogand VL, Storck G. Die oxydation von polyepoxiden mit molekularem sauerstoff. Die Makromolekulare Chemie. 1966;91:50–73.
Donbrow M. Stability of polyoxyethylene chain in non ionic surfactants. In: Schick MJ, editor. Nonionic surfactants: physical chemistry, vol. 23. new york: CRC; 1987. p. 1135.
Yao J, Dokuru DK, Noestheden M, Park SS, Kerwin BA, Jona J, et al. A quantitative kinetic study of polysorbate autoxidation: the role of unsaturated fatty acid ester substituents. Pharm Res-Dord. 2009;26:2303–13.
Zhou Y, Woo LK, Angelici RJ. Solid acid catalysis of tandem isomerization-lactonization of olefinic acids. Applied Catalysis A. 2007.
Shepherdand IS, Showell JS. The mechanism of the aqueous perchloric acid isomerization of oleic acid to -stearolactone. Journal of the American Oil Chemists’ Society. 1969;46:479–81.
Arudi RL, Sutherland MW, Bielski BH. Purification of oleic acid and linoleic acid. J Lipid Res. 1983;24:485.
Li S, Schöneich C, Borchardt RT. Chemical instability of protein pharmaceuticals: mechanisms of oxidation and strategies for stabilization. Biotechnol Bioeng. 2004;48:490–500.
Tomita M, Irie M, Ukita T. Sensitized photooxidation of histidine and its derivatives. Products and mechanism of the reaction. Biochemistry. 1969;8:5149–60.
Müller R, Karle A, Vogt A, Kropshofer H, Ross A, Maeder K, et al. Evaluation of the immuno-stimulatory potential of stopper extractables and leachables by using dendritic cells as readout. J Pharm Sci. 2009;98:3548–61.
Porter NA, Cladwell SE, Mills KA. Mechanisms of free-radical oxidation of unsaturated lipids. Amer Oil Chemists Soc. 1995;30:277–90.
O’Brien EP, Dima RI, Brooks B, Thirumalai D. Interactions between hydrophobic and ionic solutes in aqueous guanidinium chloride and urea solutions: lessons for protein denaturation mechanism. J Am Chem Soc. 2007;129:7346–53.
Mirgorodskaya AB, Yatskevich EI, Zakharova LY. The solubilization of fatty acids in systems based on block copolymers and nonionic surfactants. Russ J Phys Chem A. 2010;84:2066–70.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Balz Fischer and B. Gessier for SBSE-GC-MS measurements, Dr. Heribert Dolt and Dr. Siegfred Stolz for FT-MS measurements, Dr. Alfred Ross for NMR measurements, Dr. Monira Siam for FT-IR measurements, Dr. Andreas Staempfli for GC-MS measurements, and Christian Lehrmayer, Thomas Steffen and Martin Weiss for their help in the experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishore, R.S.K., Kiese, S., Fischer, S. et al. The Degradation of Polysorbates 20 and 80 and its Potential Impact on the Stability of Biotherapeutics. Pharm Res 28, 1194–1210 (2011). https://doi.org/10.1007/s11095-011-0385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0385-x