Abstract
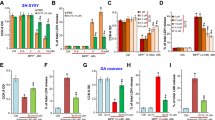
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with a prevalence of 1–2% in people over the age of 50. Mitochondrial dysfunction occurred in PD patients showing a 15–30% loss of activity in complex I. Asiatic acid (AA), a triterpenoid, is an antioxidant and used for depression treatment, but the effect of AA against PD-like damage has never been reported. In the present study, we investigated the protective effects of AA against H2O2 or rotenone-induced cellular injury and mitochondrial dysfunction in SH-SY5Y cells. Mitochondrial membrane potential (MMP) and the expression of voltage-dependent anion channel (VDAC) were detected with or without AA pretreatment following cellular injury to address the possible mechanisms of AA neuroprotection. The results showed that pre-treatment of AA (0.01–100 nM) protected cells against the toxicity induced by rotenone or H2O2. In addition, MMP dissipation occurred following the exposure of rotenone, which could be prevented by AA treatment. More interestingly, pre-administration of AA inhibited the elevation of VDAC mRNA and protein levels induced by rotenone(100 nM) or H2O2 (300 μM).These data indicate that AA could protect neuronal cells against mitochondrial dysfunctional injury and suggest that AA might be developed as an agent for PD prevention or therapy.




Similar content being viewed by others
References
Shastry BS (2001) Parkinson disease: etiology, pathogenesis and future of gene therapy. Neurosci Res 41:5–12. doi:10.1016/S0168-0102(01)00254-1
Yuan H, Zheng JC, Liu P et al (2007) Pathogenesis of Parkinson’s disease: oxidative stress, environmental impact factors and inflammatory processes. Neurosci Bull 23:125–130. doi:10.1007/s12264-007-0018-x
Ramsey CP, Giasson BI (2007) Role of mitochondrial dysfunction in Parkinson’s disease-implications for treatment. Drugs Aging 24:95–105. doi:10.2165/00002512-200724020-00002
Tansey MG, McCoy MK, Frank-Cannon TC (2007) Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 208:1–25. doi:10.1016/j.expneurol.2007.07.004
Bowling AC, Beal MF (1995) Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci 56:1151–1171. doi:10.1016/0024-3205(95)00055-B
Bindoff LA, Birch-Machin MA, Cartlidge NEF et al (1991) Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. J Neurol Sci 104:203–208. doi:10.1016/0022-510X(91)90311-T
Blin O, Desnuelle C, Rascol O et al (1994) Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J Neurol Sci 125:95–101. doi:10.1016/0022-510X(94)90248-8
Janetzky B, Hauck S, Youdim MBH et al (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett 169:126–128. doi:10.1016/0304-3940(94)90372-7
Benecke R, Strumper P, Weiss H (1993) Electron transfer complexes I and IV of platelets are abnormal in Parkinson’s disease but normal in Parkinson-plus syndromes. Brain 116:1451–1463. doi:10.1093/brain/116.6.1451
Cardellach F, Marti MJ, Fernandez-Sola J et al (1993) Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology 43:2170–2172
Cooper JM, Daniel SE, Marsden CD et al (1995) L-dihydroxyphenylalanine and complex I deficiency in Parkinson’s disease brain. Mov Disord 10:295–297. doi:10.1002/mds.870100311
Parker WJ, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–723. doi:10.1002/ana.410260606
Armstrong JS (2007) Mitochondrial medicine: Pharmacological targeting of mitochondria in disease. Br J Pharmacol 151:1154–1165. doi:10.1038/sj.bjp.0707288
Liu JK, Ames BN (2005) Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci 8:67–89. doi:10.1080/10284150500047161
Green D, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. doi:10.1126/science.1099320
Armstrong JS (2006) The role of the mitochondrial permeability transition in cell death. Mitochondrion 6:225–234. doi:10.1016/j.mito.2006.07.006
Kim J, He L, Qian T et al (2003) Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med 3:527–535. doi:10.2174/1566524033479564
Godbole A, Varghese J, Sarin A et al (2003) VDAC is a conserved element of death pathways in plant and animal systems. Biochim Biophys Acta 1642:87–96. doi:10.1016/S0167-4889(03)00102-2
Lemasters JJ, Nieminen AL, Qian T et al (1997) The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 174:159–165. doi:10.1023/A:1006827601337
Zou H, Li Y, Liu X et al (1999) An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556. doi:10.1074/jbc.274.17.11549
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Wattanathorn J, Mator L, Muchimapura S et al (2007) Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol 116:325–332. doi:10.1016/j.jep.2007.11.038
Shetty BS, Udupa SL, Udupa AL et al (2006) Effect of Centella asiatica L (Umbelliferae) on normal and dexamethasone-suppressed wound healing in Wistar Albino rats. Int J Low Extrem Wounds 5:137–143. doi:10.1177/1534734606291313
Gao J, Chen J, Tang XH et al (2006) Mechanism underlying mitochondrial protection of asiatic acid against hepatotoxicity in mice. J Pharm Pharmacol 58:227–233. doi:10.1211/jpp.58.2.0010
Lee Mk, Kim SR, Sung SH et al (2000) Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol 108:75–86
Mook JI, Shin JE, Yun SH et al (1999) Protective effects of asiaticoside derivatives against beta-amyloid neurotoxicity. J Neurosci Res 58:417–425. doi:10.1002/(SICI)1097-4547(19991101)58:3<417::AID-JNR7>3.0.CO;2-G
Betarbet R, Sherer TB, MacKenzie G et al (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306. doi:10.1038/81834
Sherer TB, Betarbet R, Stout AK et al (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015
Kitamura Y, Inden M, Miyamura A et al (2002) Possible involvement of both mitochondria- and endoplasmic reticulum-dependent caspase pathways in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci Lett 333:25–28. doi:10.1016/S0304-3940(02)00964-3
Ding HQ, Gao J, Zhu ZR et al (2008) Mitochondrial dysfunction enhances susceptibility to oxidative stress by down-regulation of thioredoxin in human neuroblastoma cells. Neurochem Res 33:43–50. doi:10.1007/s11064-007-9405-y
Wadia J, Chalmers-Redman RM, Ju WJ et al (1998) Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (-)-deprenyl. J Neurosci 18:932–947
Beal MF (1995) Aging, energy, and oxidative stress in neurodegenerative disease. Ann Neurol 38:357–366. doi:10.1002/ana.410380304
Schapira AH, Cooper JM, Dexter D et al (1993) Mitochondrial complex I deficiency in Parkinson’s disease. Adv Neurol 60:288–291
Isenberg JS, Klaunig JE (2000) Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol Sci 53:340–351. doi:10.1093/toxsci/53.2.340
Sherer TB, Betarbet R, Testa CM et al (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23:10756–10764
Lapointe N, St-Hilaire M, Martinoli MG et al (2004) Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J 18:717–719
Hoglinger GU, Feger J, Prigent A et al (2003) Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem 84:491–502. doi:10.1046/j.1471-4159.2003.01533.x
Marella M, Seo BB, Matsuno-Yagi A et al (2007) Mechanism of cell death caused by complex I defects in a rat dopaminergic cell line. J Biol Chem 282:24146–24156. doi:10.1074/jbc.M701819200
Moon Y, Lee KH, Park JH et al (2005) Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q(10). J Neurochem 93:1199–1208. doi:10.1111/j.1471-4159.2005.03112.x
Doran E, Halestrap AP (2000) Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem J 348:343–350. doi:10.1042/0264-6021:3480343
Gao J, Tang XH, Dou H et al (2004) Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. J Pharm Pharmacol 56:1–7. doi:10.1211/0022357044733
Lee YS, Jin D-Q, Kwon EJ et al (2002) Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett 186:83–91. doi:10.1016/S0304-3835(02)00260-4
Park BC, Bosire KO, Lee E-S et al (2005) Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett 218:81–90. doi:10.1016/j.canlet.2004.06.039
Hsu YL, Kuo PL, Lin LT et al (2005) Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther 313:333–344. doi:10.1124/jpet.104.078808
Zamzami N, Marchetti P, Castedo M et al (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377. doi:10.1084/jem.182.2.367
Mao Y-R, Jiang L, Duan Y-L et al (2007) Efficacy of catalpol as protectant against oxidative stress and mitochondrial dysfunction on rotenone-induced toxicity in mice brain. Environ Toxicol Pharmacol 23:314–318. doi:10.1016/j.etap.2006.11.012
Kokoszka JE, Waymire KG, Levy SE et al (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nat Neurosci 427:461–465
Acknowledgments
We thank Dr. Jiankang Liu, Institute for Brain Aging and Dementia, University of California, Irvine, for his careful reading and editing of this manuscript. This work was supported by Excellent Young Teachers Program of MOE and grants from Jiangsu University (07JDG012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Akitane Mori.
Rights and permissions
About this article
Cite this article
Xiong, Y., Ding, H., Xu, M. et al. Protective Effects of Asiatic Acid on Rotenone- or H2O2-Induced Injury in SH-SY5Y Cells. Neurochem Res 34, 746–754 (2009). https://doi.org/10.1007/s11064-008-9844-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9844-0