Abstract
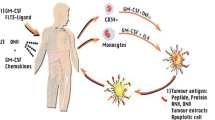
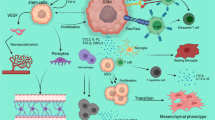
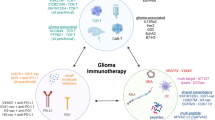
Advancements in immunotherapeutics promise new possibilities for the creation of glioblastoma (GBM) treatment options. Ongoing work in cancer stem cell biology has progressively elucidated the role of this tumor sub-population in oncogenesis and has distinguished them as prime therapeutic targets. Current clinical trials take a multifaceted approach with the intention of harnessing the intrinsic cytotoxic capabilities of the immune system to directly target glioblastoma cancer stem cells (gCSC) or indirectly disrupt their stromal microenvironment. Monoclonal antibodies (mAbs), dendritic cell (DC) vaccines, and chimeric antigen receptor (CAR) T cell therapies have emerged as the most common approaches, with particular iterations incorporating cancer stem cell antigenic markers in their treatment designs. Ongoing work to determine the comprehensive antigenic profile of the gCSC in conjunction with efforts to counter the immunosuppressive tumor microenvironment holds much promise in future immunotherapeutic strategies against GBM. Given recent advancements in these fields, we believe there is tremendous potential to improve outcomes of GBM patients in the continuing evolution of immunotherapies targeted to cancer stem cell populations in GBM.

Similar content being viewed by others
References
Vescovi AL, Galli R, Reynolds BA (2006) Brain tumour stem cells. Nat Rev Cancer 6:425–436. doi:10.1038/nrc1889
Chester C, Marabelle A, Houot R, Kohrt HE (2015) Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumors. Curr Opin Immunol 33C:1–8. doi:10.1016/j.coi.2014.12.010
Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA (2002) Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206. doi:10.1002/glia.10094
Beier D, Schulz JB, Beier CP (2011) Chemoresistance of glioblastoma cancer stem cells–much more complex than expected. Mol Cancer 10:128. doi:10.1186/1476-4598-10-128
Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF (2005) Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8:119–130. doi:10.1016/j.ccr.2005.07.004
Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncol 9:424–429. doi:10.1215/15228517-2007-023
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. doi:10.1038/nature03128
Nakano I (2014) Stem cell signature in glioblastoma: therapeutic development for a moving target. J Neurosurg. doi:10.3171/2014.9.JNS132253
Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, Wang M, Hu B, Cheng SY, Sobol RW, Nakano I (2013) Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA 110:8644–8649. doi:10.1073/pnas.1221478110
Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WF, Boddeke HW, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. doi:10.1016/j.ccr.2013.08.001
Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G (2009) SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 27:40–48. doi:10.1634/stemcells.2008-0493
Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN (2008) Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res 68:6043–6048. doi:10.1158/0008-5472.CAN-08-1079
Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN (2008) Identification of A2B5 + CD133- tumor-initiating cells in adult human gliomas. Neurosurgery 62:505–514. doi:10.1227/01.neu.0000316019.28421.95 discussion 514-505
Emlet DR, Gupta P, Holgado-Madruga M, Del Vecchio CA, Mitra SS, Han SY, Li G, Jensen KC, Vogel H, Xu LW, Skirboll SS, Wong AJ (2014) Targeting a glioblastoma cancer stem-cell population defined by EGF receptor variant III. Cancer Res 74:1238–1249. doi:10.1158/0008-5472.CAN-13-1407
Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, Ouafik L, Figarella-Branger D (2010) A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol 20:211–221. doi:10.1111/j.1750-3639.2009.00269.x
Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S (2010) HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 16:474–485. doi:10.1158/1078-0432.CCR-09-1322
Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, Barish ME, Forman SJ, Jensen MC (2012) Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res 18:2199–2209. doi:10.1158/1078-0432.CCR-11-1669
Hickey WF, Hsu BL, Kimura H (1991) T-lymphocyte entry into the central nervous system. J Neurosci Res 28:254–260. doi:10.1002/jnr.490280213
Wilson EH, Weninger W, Hunter CA (2010) Trafficking of immune cells in the central nervous system. J Clin Invest 120:1368–1379. doi:10.1172/JCI41911
Tambuyzer BR, Ponsaerts P, Nouwen EJ (2009) Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol 85:352–370. doi:10.1189/jlb.0608385
Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flugel-Koch C, Flugel A (2012) T cells become licensed in the lung to enter the central nervous system. Nature 488:675–679. doi:10.1038/nature11337
Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR (2005) Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22:175–184. doi:10.1016/j.immuni.2004.12.008
Davies DC (2002) Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat 200:639–646
Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M (2011) Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin dev Immunol 2011:732413. doi:10.1155/2011/732413
Avril T, Vauleon E, Tanguy-Royer S, Mosser J, Quillien V (2011) Mechanisms of immunomodulation in human glioblastoma. Immunotherapy 3:42–44. doi:10.2217/imt.11.39
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE 2nd, Bigner DD, Dranoff G, Sampson JH (2006) Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 66:3294–3302. doi:10.1158/0008-5472.CAN-05-3773
Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, Campoli M, Ferrone S (2005) Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res 11:8304–8311. doi:10.1158/1078-0432.CCR-04-2588
Huettner C, Paulus W, Roggendorf W (1995) Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol 146:317–322
Sawamura Y, Diserens AC, de Tribolet N (1990) In vitro prostaglandin E2 production by glioblastoma cells and its effect on interleukin-2 activation of oncolytic lymphocytes. J Neurooncol 9:125–130
Roth P, Mittelbronn M, Wick W, Meyermann R, Tatagiba M, Weller M (2007) Malignant glioma cells counteract antitumor immune responses through expression of lectin-like transcript-1. Cancer Res 67:3540–3544. doi:10.1158/0008-5472.CAN-06-4783
Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, Melillo G, Priebe W, Heimberger AB (2011) Hypoxia potentiates glioma-mediated immunosuppression. PloS one 6:e16195. doi:10.1371/journal.pone.0016195
Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB (2010) Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncol 12:1113–1125. doi:10.1093/neuonc/noq082
Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216:15–24. doi:10.1002/path.2370
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12:278–287. doi:10.1038/nrc3236
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82. doi:10.1016/j.ccr.2006.11.020
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA 109:6662–6667. doi:10.1073/pnas.1121623109
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, Weissman IL (2013) Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA 110:11103–11108. doi:10.1073/pnas.1305569110
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13:84–88. doi:10.1038/nm1517
Wilmotte R, Burkhardt K, Kindler V, Belkouch MC, Dussex G, Tribolet N, Walker PR, Dietrich PY (2005) B7-homolog 1 expression by human glioma: a new mechanism of immune evasion. NeuroReport 16:1081–1085
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144. doi:10.1056/NEJMoa1305133
Krummel MF, Allison JP (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183:2533–2540
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, Li G, Liau LM, Prins RM (2012) Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS ONE 7:e32614. doi:10.1371/journal.pone.0032614
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. doi:10.1056/NEJMoa1003466
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189–2199. doi:10.1056/NEJMoa1406498
Dahlrot RH, Hermansen SK, Hansen S, Kristensen BW (2013) What is the clinical value of cancer stem cell markers in gliomas? Int J Clin Exp Pathol 6:334–348
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP (2007) CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67:4010–4015. doi:10.1158/0008-5472.CAN-06-4180
Palucka AK, Laupeze B, Aspord C, Saito H, Jego G, Fay J, Paczesny S, Pascual V, Banchereau J (2005) Immunotherapy via dendritic cells. Adv Exp Med Biol 560:105–114. doi:10.1007/0-387-24180-9_14
Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179. doi:10.1038/sj.bjc.6601268
Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167. doi:10.1158/1078-0432.CCR-05-0120
Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979. doi:10.1158/0008-5472.CAN-03-3505
Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS (2009) Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 27:1734–1740. doi:10.1002/stem.102
Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F, Bruzzone MG, Finocchiaro G (2006) Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res 66:10247–10252. doi:10.1158/0008-5472.CAN-06-2048
(2013) ImmunoCellular Therapeutics Phase II Study Demonstrates That Glioblastoma Patients Live Longer Without Disease Progression When Treated With ICT-107
Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, Saeboe-Larssen S, Sandberg C, Brinchmann JE, Helseth E, Rasmussen AM, Lote K, Aamdal S, Gaudernack G, Kvalheim G, Langmoen IA (2013) Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 62:1499–1509. doi:10.1007/s00262-013-1453-3
Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, Zhou J, Sahin A, Carter BS, Brem H, Junghans RP, Sampath P (2012) Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res 18:5949–5960. doi:10.1158/1078-0432.CCR-12-0319
Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, Zhao YY, Zhao LM, Cui J, Gupta P, Wong AJ, Han SY (2013) Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol 6:33. doi:10.1186/1756-8722-6-33
Wang LF, Fokas E, Bieker M, Rose F, Rexin P, Zhu Y, Pagenstecher A, Engenhart-Cabillic R, An HX (2008) Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep 19:151–156
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. doi:10.1200/JCO.2010.28.6963
Acknowledgments
S.H.C. is the Tashia and John Morgridge Faculty Scholar of the Children’s Health Research Institute at Stanford, and the Ty Louis Campbell Foundation St. Baldrick’s Scholar. We would like to thank the Ludwig Center for Cancer Stem Cell Research and Medicine, the Stanford University School of Medicine Medical Scholars Research Program, the American Brain Tumor Association, Alex’s Lemonade Stand Foundation for Childhood Cancer, the St. Baldrick’s Foundation, the CureSearch for Children’s Cancer Foundation, and the Children’s Health Research Institute at Stanford for their support in the preparation of this manuscript. We would like to thank Sharareh Gholamin, M.D., Suzana A. Kahn, P.h.D., and Bahauddeen Alrfaei, Ph.D. for their insightful suggestions and discussion in the preparation of this review.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Rogelio Esparza and Tej D. Azad have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Esparza, R., Azad, T.D., Feroze, A.H. et al. Glioblastoma stem cells and stem cell-targeting immunotherapies. J Neurooncol 123, 449–457 (2015). https://doi.org/10.1007/s11060-015-1729-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1729-x