Abstract
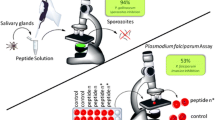
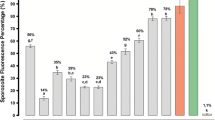
Malaria is caused by the protozoa Plasmodium and is responsible for approximately one million deaths annually. The antimalarial effects of angiotensin II and its analogs against Plasmodium gallinaceum and falciparum have recently been reported. Here, 12 angiotensin II restricted analogs that contain i − (i + 2), i − (i + 3) and i − (i + 4) lactam bridges were synthesized to analyze their effect on antiplasmodial activity. To accomplish this, peptides containing two amino acid residues (aspartic or glutamic acids and lysine or ornithine), were synthesized by the t-Boc solid phase method, purified by liquid chromatography, and characterized by mass spectrometry, and conformational studies were performed by circular dichroism. The results indicate that some of the analogs had anti-plasmodium activity similar to angiotensin II (88 % activity). Among those, eight compounds exhibited high activity (>70 %), measured by fluorescence microscopy. The analogs with smaller lactam rings and an aspartic acid residue as the bridgehead element had lower levels of lytic activity. The results obtained with the new restricted analogs showed that the insertion position (near the N-terminus), the ring size, and the number of residues between the rings are as important as the components of lactam bridge, regardless of their chirality. The circular dichroism studies suggest that the active analogs, and native angiotensin II, adopt a β-fold conformation in different solutions. In conclusion, this approach provides insight for understanding the effects of restricting the ring size and position on the bioactivity of angiotensin II and provides a new direction for the design of potential chemotherapeutic agents.




Similar content being viewed by others
References
Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA (2011) Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA 108:E1214–E1223. doi:10.1073/pnas.1112037108
Ahn JM, Gitu PM, Medeiros M, Swift JR, Trivedi D, Hruby VJ (2001) A new approach to search for the bioactive conformation of glucagon: positional cyclization scanning. J Med Chem 44:3109–3116. doi:10.1021/jm010091q
Akaddar A, Doderer-Lang C, Marzahn M, Delalande F, Mousli M, Helle K, Van Dorsselaer A, Aunis D, Dunn B, Metz-Boutigue M-H, Candolfi E (2010) Catestatin, an endogenous Chromogranin A-derived peptide, inhibits in vitro growth of Plasmodium falciparum. Cell Mol Life Sci 67:1005–1015. doi:10.1007/s00018-009-0235-8
Augagneur Y, Wesolowski D, Tae HS, Altman S, Ben Mamoun C (2012) Gene selective mRNA cleavage inhibits the development of Plasmodium falciparum. Proc Natl Acad Sci USA 109(16):6235–6240. doi:10.1073/pnas.1203516109
Baker DA (2010) Malaria gametocytogenesis. Mol Biochem Parasit 172:57–65. doi:10.1016/j.molbiopara.2010.03.019
Beaulieu P, Lambert C (1998) Peptidic regulation of heart rate and interactions with the autonomic nervous system. Cardiovasc Res 37:578–585. doi:10.1016/S0008-6363(97)00305-2
Bittermann H, Einsiedel J, Hubner H, Gmeiner P (2004) Evaluation of lactam-bridged neurotensin analogues adjusting psi(Pro(10)) close to the experimentally derived bioactive conformation of NT(8-13). J Med Chem 47:5587–5590. doi:10.1021/jm049644y
Carpenter KA, Wilkes BC, Scheller PW (1998) The octapeptide angiotensin II adopts a well-defined structure in a phospholipid environment. Eur J Biochem 251:448–453. doi:10.1046/j.1432-1327.1998.2510448.x
Castro B, Dormoy JR, Dourtoglou B, Evin G, Selve C, Ziegler JC (1976) Peptide coupling reagents. 6. Novel, cheaper preparation of benzotriazolyloxytris[dimethylamino]phosphonium hexafluorophsphate (BOP reagent). Synthesis-Stuttgart 11:751–752 ISSN: 0039-7881
Chamlian M, Bastos EL, Maciel C, Capurro ML, Miranda A, Silva AF, Torres MDT, Oliveira VX Jr (2013) A study of the anti-plasmodium activity of angiotensin II analogs. J Pept Sci 19:575–580. doi:10.1002/psc.2534
Chavain N, Biot C (2010) Organometallic complexes: new tools for chemotherapy. Curr Med Chem 17(25):2729–2745
Cho NJ, Asher SA (1996) UV resonance Raman and absorption studies of angiotensin II conformation in lipid environments. Bioespectroscopy 2:71–82. doi:10.1002/(SICI)1520-6343
Cushman JA, Mishra PK, Bothner-By AA, Khosla MS (1992) Conformations in solution of angiotensin II in aqueous solution, and its 1–7 and 1–6 fragments. Biopolymers 32:1163–1171. doi:10.1002/bip.360320905
Devappa RK, Makkar HPS, Becker K (2010) Nutritional, biochemical, and pharmaceutical potential of proteins and peptides from Jatropha: review. J Agric Food Chem 58:6543–6555. doi:10.1021/jf100003z
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ (2009) Artemisinin resistance in Plasmodium falciparum Malaria. New Engl J Med 361:455–467
Dong M, Te JA, Xu X, Wang J, Pinon DI, Storjohann L, Bordner AJ, Miller LJ (2011a) Lactam constraints provide insights into the receptor-bound conformation of secretin and stabilize a receptor Antagonist. Biochemistry 50:8181–8192. doi:10.1021/bi2008036
Dong Y, Das S, Cirimotich C, Souza-Neto JA, McLean KJ, Dimopoulos G (2011b) Engineered anopheles immunity to Plasmodium infection. PLoS Pathog 7:e1002458. doi:10.1371/journal.ppat.1002458
Fasman GD (1996) Circular dichroism and the conformational analysis of biomolecules. Plenum Press, New York
Fyhrquist F, Saijonmaa O (2008) Renin-angiotensin system revisited. J Intern Med 264:224–236. doi:10.1111/j.1365-2796.2008.01981.x
Gao B, Xu J, Rodriguez MC, Lanz-Mendoza H, Hernández-Rivas R, Du W, Zhu S (2010) Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 92:350–359. doi:10.1016/j.biochi.2010.010.011
Garcia LS (2010) Malaria. Clin Lab Med 30:93–129. doi:10.1016/j.cll.2009.10.001
Greef D, Fermandjian S, Fromageot P, Khosla MC, Smeby RR, Bumpus FM (1976) Circular-dichroism spectra of truncated and other analogs of angiotensin II. Eur J Biochem 61:297–305. doi:10.1111/j.1432-1033.1976.tb10022.x
Hruby VJ (1982) Conformtional Restrictions of Biologically Active Peptides Via Amino Acid Side Chain Groups. Life Sci 31:189–199. doi:10.1016/0024-3205(82)90578-1
Juliano L, Paiva ACM (1974) Conformation of angiotensin II in aqueous solutions. Tritation of several peptide analogs and homologs. Biochemistry 13:2445–2450. doi:10.1021/bi00708a032
Kaiser E, Colescot RL, Bossinge CD, Cook PI (1970) Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal Biochem 34(2):595. doi:10.1016/0003-2697(70)90146-6
Kortagere S, Welsh WJ, Morrisey JM, Daly T, Ejigiri I, Sinnis P, Vaidya AB, Bergman LW (2010) Structure-based design of novel small-molecule inhibitors of Plasmodium falciparum. J Chem Inf Model 50(5):840–849. doi:10.1021/ci100039k
Kumar NV, Wemmer DE, Kallenbach NR (1988) Structure of P401 (mast cell degranulating peptide) in solution. Biophys Chem 31:113–119. doi:10.1016/0301-4622(88)80015-2
Kuter D, Chibale K, Egan TJ (2011) Linear free energy relationships predict coordination and p-stacking interactions of small molecules with ferriprotoporphyrin IX. J Inorg Biochem 105:684–692. doi:10.1016/j.jinorgbio.2011.02.008
Kyte J, Doolittle RF (1982) A simple method for displaying the hydrophatic character of a protein. J Mol Biol 157:105–132. doi:10.1016/0022-2836(82)90515-0
Lin SH, Konishi Y, Denton ME, Scheraga HA (1984) Influence of an extrinsic cross-link on the folding pathway of ribonuclease A. conformational and thermodynamic analysis of cross-linked (lysine7-lysine41)-ribonuclease A. Biochemistry 23:5504–5512. doi:10.1021/bi00318a019
Longmuir K, Robertson R, Haynes S, Baratta J, Waring A (2006) Effective targeting of liposomes to liver and hepatocytes in vivo by incorporation of a Plasmodium amino acid sequence. Pharm Res 23:759–769
Maciel C, Oliveira VX, Fázio MA, Nacif-Pimenta R, Miranda A, Pimenta PF, Capurro ML (2008) Anti-plasmodium Activity of Angiotensin II and Related Synthetic Peptides. PLoS ONE 3:e3296. doi:10.1371/journal.pone.0003296
Mitchell AR, Kent SBH, Engelhard M, Merrifield RB (1978) New synthetic route to tert-butyloxycarbonylaminoacyl-4-(oxymethyl)phenylacetamidomethyl-resin, an improved support for solid-phase peptide-synthesis. J Org Chem 43(14):2845–2852. doi:10.1021/jo00408a022
Moll GN, Konings WN, Driessen AJM (1999) Bacteriocins: mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 76:185–198. doi:10.1023/A:1002002718501
Moreira LA, Ghosh AK, Abraham EG, Jacobs-Lorena M (2002) Genetic transformation of mosquitoes: a quest for malaria control. Int J Parasitol 32:1599–1605. doi:10.1016/S0020-7519(02)00188-1
Mosberg HI (1999) Complementarity of delta opioid ligand pharmacophore and receptor models. Biopolymers 51:426–439. doi:10.1002/(SICI)1097-0282
Munstermann LE, Conn JE (1997) Systematics of mosquito disease vectors (Diptera, Culicidae): impact of molecular biology and cladistic analysis. Annu Rev Entomol 42:351–369. doi:10.1146/annurev.ento.42.1.351
Neves DP, Melo AL, Linardi PM, Vitor RWA (2005) Parasitologia humana, 11th edn. Atheneu, Rio de Janeiro
Oliveira VX, Fázio MA, Silva AF, Campana PT, Pesquero JB, Santos EL, Costa-Neto CM, Miranda A (2011) Biological and conformational evaluation of angiotensin II lactam bridge containing analogues. Regul Peptides 172:1–7. doi:10.1016/j.regpep.2011.05.015
Orjuela-Sanchez P, Karunaweera N, da Silva-Nunes M, da Silva N, Scopel K, Goncalves R, Amaratunga C, Sa J, Socheat D, Fairhust R (2010) Single-nucleotide polymorphism, linkage disequilibrium and geographic structure in the malaria parasite Plasmodium vivax: prospects for genome-wide association studies. BMC Genet 11:65. doi:10.1186/1471-2156-11-65
Patarroyo ME, Patarroyo MA (2008) Emerging rules for subunit-based, multiantigenic, multi stage chemically synthesized vaccines. Acc Chem Res 41(3):377–386. doi:10.1021/ar700120t
Pease JH, Wemmer DE (1988) Solution structure of apamin determined by nuclear magnetic resonance and distance geometry. Biochemistry 27:8491–8498. doi:10.1021/bi00422a029
Pellegrini M, Mierke DF (1999) Structural characterization of peptide hormone/receptor interactions by NMR spectroscopy. Biopolymers 51:208–220. doi:10.1002/(SICI)1097-0282
Piriou F, Lintner K, Fermandjian S, Fromageot P, Khosla MC, Smeby RR, Bumpus FM (1980) Amino acid side chain conformation in angiotensin II and analogs: correlated results of circular dichroism and 1H nuclear magnetic resonance. Biochemistry 77:82–86. doi:10.1073/pnas.77.1.82
Polevaya L, Mavromoustakos T, Zoumboulakis P, Grdadolnik SG, Roumelioti P, Giatas N, Mutule I, Keivish T, Vlahakos DV, Iliodromitis EK, Kremastinos DT, Matsoukas J (2001) Synthesis and study of a cyclic angiotensin II antagonist analogue reveals the role of π*–π* interactions in the C-terminal aromatic residue for agonist activity and its structure resemblance with AT1 non-peptide antagonists. Bioorg Med Chem 9:1639–1647. doi:10.1016/S0968-0896(01)00059-1
Portela MJ, Moreira R, Valente E, Constantino L, Iley J, Pinto J, Rosa R, Cravo P, do Rosario VE (1999) Dipeptide derivatives of primaquine as transmission-blocking antimalarials: effect of aliphatic side-hain acylation on the gametocytocidal activity and on the formation of carboxyprimaquine in rat liver homogenates. Pharm Res 16(6):949–955. doi:10.1023/A:1018922425551
Printz MP, Williams HP, Craig LC (1972) Evidence for the presence of hydrogen-bonded secondary structure in angiotensin II in aqueous solution. PNAS 69:378–382. doi:10.1073/pnas.69.2.378
Rathi B, Singh AK, Kishan R, Singh N, Latha N, Srinivasan S, Pandey KC, Tiwari HK, Singh BK (2013) Functionalized hydroxyethylamine based peptide nanostructures as potential inhibitors of falcipain-3, an essential proteases of Plasmodium falciparum. Bioorg Med Chem 21:5503–5509. doi:10.1016/j.bmc.2013.05.052
Rizo J, Geirasch LM (1992) Constrained peptides: models of bioactive peptides and proteins structures. Ann Rev Biochem 61:387–418. doi:10.1146/annurev.bi.61.070192.002131
Robertson RT, Baratta JL, Haynes SM, Longmuir KJ (2008) Liposomes incorporating a Plasmodium amino acid sequence target heparan sulfate binding sites in liver. J Pharm Sci 97:3257–3273. doi:10.1002/jps.21211
Spolar RS, Jr Record MT (1994) Coupling of local folding to site-specific binding of proteins to DNA. Science 263:777–784. doi:10.1126/science.8303294
Stewart JM, Young JD (1984) Solid-phase peptide synthesis. Pierce Chemical Co, Rockford
Tang TH, Salas A, Ali-Tammam M, Martinez M, Lanza M, Arroyo E, Rubio J (2010) First case of detection of Plasmodium knowlesi in Spain by real time PCR in a traveller from Southeast Asia. Malar J 9:219. doi:10.1186/1475-2875-9-219
Teixeira C, Gomes JR, Gomes P (2011) Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr Med Chem 18:1555-1572 ISSN: 0929-8673
Thathy V, Severson DW, Christensen BM (1994) Reinterpretation of the genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. J Parasitol 80:705–712. doi:10.2307/3283249
Tzakos AG, Bonvin AMJJ, Troganis AN, Cordopatis P, Amzel ML, Gerothanassis IP, van Nuland NAJ (2003) On the molecular basis of the recognition of angiotensin II (AII) - NMR structure of AII in solution compared with the X-ray structure of AII bound to the mAb Fab131. Eur J Biochem 270(5):849–860. doi:10.1046/j.1432-1033.2003.03441.x
Tzakos AG, Gerothanassis IP, Troganis AN (2004) On the structural basis of the hypertensive properties of angiotensin II: a solved mystery or a controversial issue? Curr Top Med Chem 4(4):431–444. doi:10.2174/1568026043451375
Vijayaraghavan K, Deedwania P (2011) Renin-angiotensin-aldosterone blockade for cardiovascular disease prevention. Cardiol Clin 29:137–156. doi:10.1016/j.ccl.2010.11.003
Walker CS, Steel D, Jacobsen RB, Lirazan MB, Cruz LJ, Hooper D, Shetty R, DelaCruz RC, Nielsen JS, Zhou LM, Bandyopadhyay P, Craig AG, Olivera BM (1999) The T-superfamily of conotoxins. J Biol Chem 274:30664–30671. doi:10.1074/jbc.274.43.30664
Wells TN, Alonso PL, Gutteridge WE (2009) New medicine to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov 8:879–891. doi:10.1038/nrd2972
Whicher JR, Florova G, Sydor PK, Singh R, Alhamadsheh M, Challis GL, Reynolds KA, Smith JL (2011) Structure and function of the Red J protein, a thioesterase from the prodiginine biosynthetic pathway in streptomyces coelicolor. J Biol Chem 286(25):22558–22569. doi:10.1074/jbc.M110.213512
Wong NLM, Fok A, Wang MH (2003) Endothelin (ET) mediates angiotensin II (ANG II) induced upregulation of vasopressin (AVP) mRNA in the inner medullary-collecting duct (IMCD). J Am Soc Nephrol 543A-A. doi: 10.1053/meta.2003.50047
Yandara N, Pastorin G, Prato M, Bianco A, Patarroyo ME, Manuel Lozano J (2008) Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide-carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine 26:5864–5873. doi:10.1016/j.vaccine.2008.08.014
Acknowledgments
This research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo, (FAPESP, VXO #2011/10823-9, MDTT #2011/15083-3, AFS #2011/11448-2). This study was carried out in strict accordance with the protocol approved by the Committee on the Ethics in Research of the Universidade Federal de São Paulo (Permit Number: 2013/479357) and Universidade de São Paulo (Permit number: 133). All surgery was performed under anesthesia, and all efforts were made to minimize animal suffering and all institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
Marcelo D. T. Torres, Adriana F. Silva, Flavio L. Alves, Margareth L. Capurro, Antonio Miranda, and Vani X. Oliveira Jr. declare that have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Der Torossian Torres, M., Silva, A.F., Alves, F.L. et al. The Importance of Ring Size and Position for the Antiplasmodial Activity of Angiotensin II Restricted Analogs. Int J Pept Res Ther 20, 277–287 (2014). https://doi.org/10.1007/s10989-014-9392-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-014-9392-1