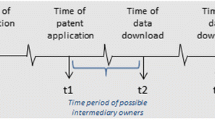
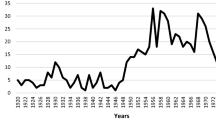
Abstract
Drawing on histories of technological innovation originating from research by faculty at The Pennsylvania State University and Johns Hopkins University, this paper presents evidence for a “technology” as well as an “intellectual property rights” research approach to the commercialization of academic patents. By describing how inventor and firm activities and strategies affect the technical development and commercial positioning of university patents, a technology focus adds depth to the general proposition that university patents are embryonic technologies. It likewise serves as an analytical probe to reconsider other mainstream propositions about university technology transfer.
Similar content being viewed by others
Notes
A humanitarian device exemption application is similar to an FDA premarket approval application in requiring reasonable assurance of safety and probable benefit. The exemption pertains to the small size of the potential population to benefit from the device and to smaller size trials concerning the device’s effectiveness.
Throughout these years, PSU researchers at the Hershey Medical Center continued their research on artificial heart technology, receiving, for example, a $5M contract from the National Heart, Lung, and Blood Institute in 2004 to develop a pediatric heart-assist device. Also, at the same time that it was engaged in continuing research related to the implantable heart, Hershey researchers were engaged in complementary work on other heart-assist devices. One such line of research led to the “LionHeart”, a completely implantable pump for use in individuals with congestive heart failure who didn’t qualify for a heart transplant. The agreement with abiomed did not preclude Hershey researchers from continuing their work with Arrow International, Pennsylvania, which had licensed this technology. In 2005, Arrow announced plans to stop manufacturing the LionHeart, describing it as “not economically viable for the firm”, having failed to sell any of the pumps in the previous 2 fiscal quarters.
Petition letter to the honorable Donna E. Shalala, March 3, 1997 from Lloyd Cutler and Birch Bayh at http://www.nih.gov/icd/od/foia/cellpro/index.htm.
NAT’L INSTS. of Health, Determination in the Case of Petition of CellPro, Inc. (Aug. 1, 1997) at http://www.nih.gov/icd/od/foia/cellpro/pdfs/foia_cellpro39.pdf.
Email correspondence from Howard Califano, Director of the Office of Technology Licensing to David Blake, dean of the Medical School (August 7, 1995: 11:22 a.m.).
Declaration of Dr. Jerry A. Hausman mentioned that CellPro repeatedly refused Baxter’s 1992 offer of a license for $750,000 and an 8% royalty.
Letter to Donna E. Shalala, Secretary of Department of Health and Human Services. 1997.
http://www.nih.gov/news/pr/aug97/nihb-01.htm. NIH determination in the case of petition of CellPro, Inc.
10 K report, Nexell, about their march in the Baxter’s cancer therapy and acquire of CellPro. http://sec.edgar-online.com/2000/03/30/15/0000898430-00-001062/Section2.asp.
http://sec.edgar-online.com/2000/03/30/15/0000898430-00-001062/Section2.asp. Nexell 10 K reprot filing March 30, 2001.
Capturing the stem cell. Winter 2004. Johns Hopkins Medicine. Promise and Progress. http://www.hopkinskimmelcancercenter.org/publications/publication.cfm?DocumentID=436&publicationID=15&publicationtypeid=1.
Schramm left JHU to head the Health Insurance Association of America and later became executive vice president of Fortis (now Assurant) and president of its health insurance operations. Schramm, was an active entrepreneur and co-founded HCIA, Inc. and Patient Choice Health Care and founded Greenspring Advisors.
MiniMed was formed as a subsidiary of Pacesetter and spun off in 1985 when Pacemaker was acquired by Siemens. Mann also started and controls the Advanced Bionics Corp., which makes implants that allow deaf people to hear, and Medical Research Group Inc., which is doing research on the artificial pancreas for MiniMed. All the companies are next to one another in Sylmar, Calif., north of Los Angeles.
In related marketing and product developments, MiniMed conducted user surveys to identify the most desirable features that people wanted to see in insulin pumps. Thus, the products were well received as they provided a set of attributes such as menu driven programming and style that consumers valued.
References
Arora, A., Fosfuri, A., & Gambardella, A. (2001). Markets for technology. Cambridge, MA: MIT Press.
Association of University Technology Managers. (2006). The better world report-technology transfer stories: 25 innovations that changed the world 2006 Edition (AUTM Better World Project).
Bar-Shalom, A., & Cook-Deegan, R. (2002). Patents and innovation in cancer therapeutics: Lessons from CellPro. The Milbank Quarterly, 80(4), 637–676.
Bercovitz, J., Feldman, M., Feller, I., & Burton, R. (2001). Organizational structure as a determinant of academic patenting and licensing behavior: An exploratory study of Duke, Johns Hopkins, and the Pennsylvania State Universities. Journal of Technology Transfer, 26, 21–35.
Civin, C., & Ware, D. R. (2001). CD34 wars: Biotech invention lessons presentation at the UVA.
Civin, C. I., et al. (1984). Antigenic analysis of hematopoieses III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-Ia cells. Journal of Immunology, 13(15), 7–165.
Colyvas, J., Crow, M., Gelijins, A., Mazzoleni, R., Nelson, R., Rosenberg, R., et al. (2002). How do University inventions get into practice. Management Science, 48, 73–89.
Edwards, D., & Langer, R. (1997, June 20). Large porous particles for pulmonary drug delivery. Science, 1868–1871.
Feldman, M., & Desrochers, P. (2003). The evolving role of research Universities in technology transfer: Lessons from the history of Johns Hopkins University. Industry and Innovation, 10, 5–24.
Feller, I. (1997). Technology transfer from Universities. In J. Smart (Ed.), Higher education: Handbooks of theory and research (vol. XII, pp. 1–42). New York: Agathon Press.
Feller, I., Ailes, C., & Roessner, J. (2002). Impacts of Research Universities on technological innovation in industry: Evidence from Engineering Research Centers. Research Policy, 31, 457–474.
Feller, I., Feldman, M., Bercovitz, J., & Burton, R. (2000). A disaggregated examination of patent and licensing behavior at three Research Universities. Paper presented at the Western Economic Association Meeting.
Garner, D. (1999). Advanced Inhalation Research, Inc. Harvard Business School Case Study 9-899-292; Revised June 20, 2000.
Geiger, R., & Sa, C. (2008). Tapping the riches of science. Cambridge, MA: Harvard University Press.
Gelijns, A., & Rosenberg, N. (1995). From the scapel to the scope: Endoscopic innovations in gastroenterology, gynecology, and surgery. In N. Rosenberg, A. Gelijns, & H. V. Dawkins (Eds.), Sources of medical technology and industry (pp. 67–96). Washington, DC: National Academy Press.
Goldbarb, Z. (2005). FDA panel weighs artificial-heart devices. Wall Street Journal.
Hancock, E. (1996). Stalking the stem cell. Johns Hopkins Magazine. June. http://www.jhu.edu/~jhumag/0400webweb/stemcell.html
Helper, S. (2000). Economists and field research: You can observe a lot just by watching. American Economic Review, 90, 228–232.
Industrial Research Institute, Inc. (2002). Industry–University intellectual property. Position Paper, External Research Directors Network.
Keiger, D. (2000). The search that paid off-big. Johns Hopkins Magazine. April. http://www.jhu.edu/~jhumag/04000web/43.html
Kerbeshian, M. (2004). Famous and infamous lawsuits over University—owned patents (UVA Patent Foundation).
Mowery, D., Nelson, R., Sampat, R., & Ziedonis, A. (2001). The growth of patening and licensing by US Universities: An assessment of the effects of the Bayh-Dole act of 1980. Research Policy, 30, 99–119.
Mowery, D., Nelson, R., Sampat, B., & Ziedonis, A. (2004). Ivory tower and industrial innovation. Stanford, CA: Stanford University Press.
Mowery, D., & Rosenberg, N. (1998). Paths of innovation. Cambridge, UK: Cambridge University Press.
Niosi, J. (2006). Introduction to the symposiuim: Universities as a source of commercial technology. Journal of Technology Transfer, 31, 399–402.
Rosenberg, N., & Nelson, R. (1994). Academic Universities and technical advance in industry. Research Policy, 19, 165–174.
Rothaermel, F., Aguing, S., & Jiang, L. (2007). University entrepreneurship: A taxonomy of the literature. Industrial and Corporate Change, 16, 691–791.
Sarns, R. (1999). History of perfusion, 1958–1995 speech to the American Academy of Cardiovascular Profusion, January 24, 1999, in the proceedings of the American Academy of Cardiovascular Perfusion.
Siegel, D., Wright, M., & Lockett, A. (2007). The rise of entrepreneurial activity at Universities: Organizational and societal implications. Industrial and Corporate Change, 4, 489–504.
Starfield, B., Weiner, J., Mumford, L., & Steinwachs, D. (1991). Ambulatory care groups: A categorization of diagnoses for research and management. Health Services Research, 26(1), 53–74.
Teece, D. (1987). Profiting from technological innovation. In D. Teece (Ed.), The competitive challenge (pp. 185–219). Cambridge, MA: Ballinger Publishing Co.
Thursby, J., & Thursby, M. (2003). Industry/University licensing: Characteristics, concerns and issues from the perspective of the buyer. Journal of Technology Transfer, 28, 207–213.
Von Hippel, E. (1976). The dominant role of the user in the scientific instrument innovation process. Research Policy, 5, 212–239.
Weick, K. (1995). What theory is not, theorizing is. Administrative Science Quarterly, 40, 385–390.
Yin, R. (2003). Case study research (3rd ed.). Thousand Oaks, CA: SAGE Publications.
Acknowledgments
Research on this paper was supported by a grant from the Andrew W. Mellon Foundation. We have benefited from earlier discussions with Janet Bercovitz and Richard Burton.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feller, I., Feldman, M. The commercialization of academic patents: black boxes, pipelines, and Rubik’s cubes. J Technol Transf 35, 597–616 (2010). https://doi.org/10.1007/s10961-009-9123-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10961-009-9123-5