Abstract
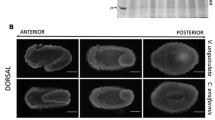
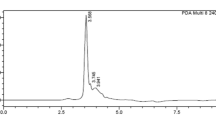
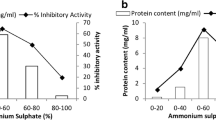
In this work, we analyzed the effects of coffee seed proteins, especially Cc-LTP1 on the larval development of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae), a bruchid pest of beans and the most important insect pest of Vigna unguiculata (L.) Walp. Artificial seed assay, which incorporated the F/0-90 fraction from Coffea canephora seeds, resulted in the reduction of oviposition and caused an inhibition of C. maculatus larval development in a dose-dependent manner. The F/0-90 fraction used at a 4 % concentration resulted in the survival of no larvae. The purified Cc-LTP1, at a concentration of 0.5 %, also demonstrated effective inhibition of larval development, reducing both females oviposition and the weight and number of larvae. Cc-LTP1 was also able to inhibit the C. maculatus gut α-amylase activity, and immunolabeling by an anti-LTP serum was observed in the midgut tissues of the C. maculatus larvae. Cc-LTP1 has shown binding affinity towards microvillar cells, endoplasmic reticulum and mitochondria, as demonstrated by micrographic images taken by a transmission electron microscope. The results from this study indicate that Cc-LTP1 has insecticidal actions toward C. maculatus and exerts anti-nutritional effects with direct actions on intestinal tissues.







Similar content being viewed by others
Abbreviations
- LTP:
-
Lipid transfer protein
- AMPs:
-
Antimicrobial peptides
- Cc-LTP1 :
-
Lipid transfer protein from Coffea canephora
- FITC:
-
Fluorescein isothiocyanate
References
Carvalho AO, Gomes VM (2007) Role of plant lipid transfer proteins in plant cell physiology—a concise review. Peptides 28:1144–1153
Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS, Park Y, Kirfel G, Komnick H (1999) Differential absorption and esterification of dietary long-chain fatty acids by larvae of the dragonfly, Aeshna cyanea. Arch Insect Biochem Physiol 40:183–193
Wong JH, Ng TB, Randy CF, Cheung XJ, Ye HX, Wang SK, Lam P, Lin YS, Fang EF, Patrick HK, Ngai LX, Ye XY (2010) Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl Microbiol Biotechnol 87:1221–1235
Carvalho AO, Gomes VM (2011) Plant defensins and defensin-like peptides—biological activities and biotechnological applications. Curr Pharm Desv 17:4270–4429
Pelegrini PB, Lay FT, Murad AM, Anderson MA, Franco OL (2008) Novel insights on the mechanism of action of α-amylase inhibitors from the plant defensin family. Proteins 73:719–729
Santos IS, Carvalho AO, Souza-Filho GA, Nascimento VV, Machado OLT, Gomes VM (2010) Purification of a defensin isolated from Vigna unguiculata seeds, its functional expression in Escherichia coli, and assessment of its insect α-amylase inhibitory activity. Protein Expr Purif 71:8–15
Bloch CJ, Richardson M (1991) A new family of small (5 kDa) protein inhibitors of insect α-amylases from seeds of sorghum (Sorghum bicolor (L) Moench) have sequence homologies with wheat γ-purothionins. FEBS 279:101–104
Osborn RW, De Samblanx GW, Thevissen K et al (1995) Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett 368:257–262
Zottich U, Da Cunha M, Carvalho AO, Dias GB, Silva NCM, Santos IS, Nascimento VV, Machado OLT, Gomes VM (2011) Purification, biochemical characterization and antifungal activity of a new lipid transfer protein (LTP) from Coffea canephora seeds with α-amylase inhibitor properties. Biochim Biophys Acta 1810:375–383
Fazuoli LC (1986) Genética e melhoramento do cafeeiro. In: Rena AB, Malavolta E, Rocha M, Yamada Y, Patafos (ed) Cultura do cafeeiro, by Piracicaba, Brazil, pp 87–113
Briones CLS, Hernández-Sotomayor SMT (2006) Coffee biotechnology. Braz J Plant Physiol 18:217–227
Svangard E, Burman R, Gunasekera S, Lövborg H, Gullbo J, Göransson U (2007) Mechanism of action of cytotoxic cyclotides: cycloviolacin O2 disrupts lipid membranes. J Nat Prod 70:643–647
Hall AE, Singh BB, Ehlers JD (1997) Cowpea breeding. Plant Breed 15:217–274
Schagger H, Von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Macedo MLR, Da Andrade SLB, Moraes RA, Xavier-Filho J (1993) Vicilin variants and the resistance of cowpea (Vigna unguiculata) seeds to the cowpea weevil (Callosobruchus maculatus). Comp Biochem Physiol 105:89–94
Bernfeld E (1955) Amylases, alpha and beta. Methods Enzymol 1:149–158
De Sá LFR, Wermelinger TT, Ribeiro ES, Gravina GA, Fernandes KVS, Xavier-Filho J, Venancio TM, Rezende GL, Oliveira AEA (2014) Effects of Phaseolus vulgaris (Fabaceae) seed coat on the embryonic and larval development of the cowpea weevil Callosobruchus maculatus (Coleoptera: Bruchidae). J Insect Physiol 60:50–57
Terra WR, Ferreira C (2005) Biochemistry of digestion. In: Gilbert LI, Iatrov K, Gill SS (eds) Comprehensive molecular insect science, biochemistry and molecular biology. Elsevier Science Publishers, Oxford, pp 171–224
Eisemann CH, Binnington KC (1994) The peritrophic membrane: its formation, structure, chemical composition and permeability in relation to vaccination against ectoparasitic arthropods. Int J Parasitol 24:15–26
Harper MS, Hopkins TL, Czapla TH (1998) Effect of wheat germ agglutinin on formation and structure of the peritrophic membrane in European corn borer (Ostrinia nubilalis) larvae. Tissue Cell 30:166–176
Habibi JE, Backus EA, Huesing JE (2000) Effects of phytohemagglutinin (PHA) on the structure of midgut epithelial cells and localization of its binding sites in western tarnished plant bug Lygus hesperus Knight. J Insect Physiol 46:611–619
Paes NS, Gerhardt IR, Coutinho MV, Yokoyama M, Santana E, Harris N, Chrispeels MJ, de Sá MFG (2000) The effect of arcelin-1 on the structure of midgut of bruchid larvae and immunolocalization of the arcelin protein. J Insect Physiol 46:393–402
Coelho MB, Macedo MLR, Marangoni S, Silva DS, Cesarino I, Mazzafera P (2010) Purification of legumin-like proteins from Coffea arabica and Coffea racemosa seeds and their insecticidal properties toward cowpea weevil (Callosobruchus maculatus) (Coleoptera: Bruchidae). J Agric Food Chem 58:3050–3055
Daly NL, Rosengren KJ, Craik DJ (2009) Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev 61:918–930
Soares EL, Freitas CDT, Oliveira JS, Sousa PAS, Sales MP, Barreto-Filho JDM, Bandeira JDM, Ramos MV (2007) Characterization and insecticidal properties of globulins and albumins from Luetzelburgia auriculata (Allemao) Ducke seeds towards Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Stored Prod Res 43:459–467
Uchôa A, Miranda M, Souza A, Gomes V, Fernandes K, Lemos F, Oliveira A, Xavier J (2009) Toxicity of hydrolyzed vicilins toward Callosobruchus maculatus and phytopathogenic fungi. J Agric Food Chem 57:8056–8061
Souza SM, Uchôa AF, Silva JR, Samuels RID, Oliveira AEA, Oliveira EM, Linhares RT, Carlos P, Silva DA (2010) The fate of vicilins, 7S storage globulins, in larvae and adult Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). J Insect Physiol 56:1130–1138
Sales MP, Pimenta PP, Paes NS, Grossi-de-Sá MF, Xavier-Filho J (2010) Vicilins (7S storage globulins) of cowpea (Vigna unguiculata) seeds bind to chitinous structures of the midgut of Callosobruchus maculatus (Coleoptera: Bruchidae) larvae. Braz J Med Biol Res 34:27–34
Van Ree R (2002) Clinical importance of non-specific lipid transfer proteins as food allergens. Biochem Soc Trans 30:910–913
Lehane HJ (1997) Peritrophic matrix structure and function. Ann Rev Entomol 42:525–550
Terra WR, Ferreira C (2005) Biochemistry of digestion. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science, vol 4. Elsevier, Oxford, pp 171–224
Drioli E, Gianfreda L, Palescandolo R, Scardi V (1976) The kinetic behaviour of enzymes gelified on ultrafiltration membranes. In: Thomas D, Kernevez JP (eds) Analysis and control of immobilised enzyme systems. North Holland, Amsterdam, p 179
Ishimoto M, Kitamura K (1989) Growth inhibitory effects of an amylase inhibitor from kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: Bruchidae). Appl Entomol Zool 24:281–286
Huesing JE, Shade RE, Chrispeels MJ, Murdock LL (1991) α-Amilase inhibitor, not phytohemagglutinin, explains resistance of common bean to cowpea weevil. Plant Physiol 96:993–996
Ishimoto M, Chrispeels MJ (1996) Protective mechanism of the Mexican bean weevil against high levels of amylase inhibitor in the common bean. Plant Physiol 111:393–401
Shade RE, Schroeder HE, Pueyo JJ, Tabe LM, Murdock LL, Higgins TJV, Chrispeels MJ (1994) Transgenic pea seeds expressing the α-amylase inhibitor of the common bean are resistant to bruchid beetles. BioTechnology 12:793–796
Schroeder HE, Gollasch S, Moore A, Tabe LM, Craig S, Hardie DC, Chispeels MJ, Spencer D, Higgins TJV (1995) Bean α-amilase inhibitor confers resistance to the pea weevil (Bruchus pisorum) in transgenic peas (Pisum sativum L.). Plant Physiol 107:1233–1239
Acknowledgments
This work is a part of the DS degree thesis of Umberto Zottich Pereira done at Universidade Estadual do Norte Fluminense. We acknowledge financial aid from the Brazilian agencies FAPERJ, CNPq, FAPES and CAPES. We are also thanks for financial aid to Dr. Viviane Veiga do Nascimento through a PNPD/CAPES fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zottich, U., Da Cunha, M., Dias, G.B. et al. The Toxicity of a Lipid Transfer Protein (Cc-LTP1) from Coffea canephora Seeds on the Larval Development of Callosobruchus maculatus (Coleoptera: Bruchidae). Protein J 33, 422–431 (2014). https://doi.org/10.1007/s10930-014-9575-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-014-9575-9