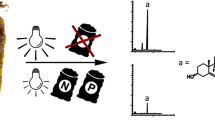
Abstract
Arthropods are incapable of synthesizing sterols de novo and thus require a dietary source to cover their physiological demands. The most prominent sterol in animal tissues is cholesterol, which is an indispensable structural component of cell membranes and serves as precursor for steroid hormones. Instead of cholesterol, plants and algae contain a variety of different phytosterols. Consequently, herbivorous arthropods have to metabolize dietary phytosterols to cholesterol to meet their requirements for growth and reproduction. Here, we investigated sterol-limited growth responses of the freshwater herbivore Daphnia magna by supplementing a sterol-free diet with increasing amounts of 10 different phytosterols and comparing thresholds for sterol-limited growth. In addition, we analyzed the sterol composition of D. magna to explore sterol metabolic constraints and bioconversion capacities. We show that dietary phytosterols strongly differ in their potential to support somatic growth of D. magna. The dietary threshold concentrations obtained by supplementing the different sterols cover a wide range (3.5–34.4 μg mg C−1) and encompass the one for cholesterol (8.9 μg mg C−1), indicating that certain phytosterols are more efficient in supporting somatic growth than cholesterol (e.g., fucosterol, brassicasterol) while others are less efficient (e.g., dihydrocholesterol, lathosterol). The dietary sterol concentration gradients revealed that the poor quality of particular sterols can be alleviated partially by increasing dietary concentrations, and that qualitative differences among sterols are most pronounced at low to moderate dietary concentrations. We infer that the dietary sterol composition has to be considered in zooplankton nutritional ecology to accurately assess potential sterol limitations under field conditions.





Similar content being viewed by others
References
Anderson TR, Hessen DO, Elser JJ, Urabe J (2005) Metabolic stoichiometry and the fate of excess carbon and nutrients in consumers. Am Nat 165:1–15
Basen T, Rothhaupt K-O, Martin-Creuzburg D (2012) Absence of sterols constrains food quality of cyanobacteria for an invasive freshwater bivalve. Oecologia 170:57–64
Bec A, Martin-Creuzburg D, von Elert E (2006) Trophic upgrading of autotrophic picoplankton by the heterotrophic nanoflagellate Paraphysomonas sp. Limnol Oceanogr 51:1699–1707
Behmer ST, Elias DO, Bernays EA (1999a) Post-ingestive feedbacks and associative learning regulate the intake of unsuitable sterols in a generalist grasshopper. J Exp Biol 202:739–748
Behmer ST, Elias DO, Grebenok RJ (1999b) Phytosterol metabolism and absorption in the generalist grasshopper, Schistocerca americana (Orthoptera : Acrididae). Arch Insect Biochem Physiol 42:13–25
Behmer ST, Grebenok RJ, Douglas AE (2011) Plant sterols and host plant suitability for a phloem-feeding insect. Funct Ecol 25:484–491
Behmer ST, Nes WD (2003) Insect sterol nutrition and physiology: A global overview. Adv Insect Physiol 31:1–72
Bertalanffy LV (1957) Quantitative laws in metabolism and growth. Q Rev Biol 32:217–231
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton
Carvalho M et al (2010) Survival strategies of a sterol auxotroph. Development 137:3675–3685
Chamberlain PM, Bull ID, Black HIJ, Ineson P, Evershed RP (2004) Lipid content and carbon assimilation in Collembola: implications for the use of compound-specific carbon isotope analysis in animal dietary studies. Oecologia 139:325–335
Clayton RB (1960) The role of intestinal symbionts in the sterol metabolism of Blattella germanica. J Biol Chem 235:3421–3425
Entchev EV, Kurzchalia TV (2005) Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol 16:175–182
Giner JL, Faraldos JA, Boyer GL (2003) Novel sterols of the toxic dinoflagellate Karenia brevis (Dinophyceae): A defensive function for unusual marine sterols? J Phycol 39:315–319
Goad LJ (1981) Sterol biosynthesis and metabolism in marine invertebrates. Pure Appl Chem 53:837–852
Grieneisen ML (1994) Recent advances in our knowledge of ecdysteroid biosynthesis in insects and crustaceans. Insect Biochem Molec Biol 24:115–132
Haines TH (2001) Do sterols reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res 40:299–324
Harvey HR, Eglinton G, O’Hara SCM, Corner EDS (1987) Biotransformation and assimilation of dietary lipids by Calanus feeding on a dinoflagellate. Geochim Cosmochim Acta 51:3031–3040
Hassett RP (2004) Supplementation of a diatom diet with cholesterol can enhance copepod egg-production rates. Limnol Oceanogr 49:488–494
Ikekawa N, Morisaki M, Fujimoto Y (1993) Sterol metabolism in insects: Dealkylation of phytosterol to cholesterol. Accounts Chem Res 26:139–146
Kanazawa A (2001) Sterols in marine invertebrates. Fisheries Sci 67:997–1007
Klein Breteler WCM, Schogt N, Baas M, Schouten S, Kraay GW (1999) Trophic upgrading of food quality by protozoans enhancing copepod growth: role of essential lipids. Mar Biol 135:191–198
Lampert W (1991) The dynamics of Daphnia in a shallow lake. Verh Int Ver Limnol 24:795–798
Lukas M, Wacker A (2014) Daphnia’s dilemma: adjustment of carbon budgets in the face of food and cholesterol limitation. J Exp Biol 217:1079–1086
Martin-Creuzburg D, Bec A, von Elert E (2005a) Trophic upgrading of picocyanobacterial carbon by ciliates for nutrition of Daphnia magna. Aquat Microb Ecol 41:271–280
Martin-Creuzburg D, Beck B, Freese HM (2011) Food quality of heterotrophic bacteria for Daphnia magna: evidence for a limitation by sterols. FEMS Microbiol Ecol 76:592–601
Martin-Creuzburg D, Sperfeld E, Wacker A (2009) Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc R Soc B 276:1805–1814
Martin-Creuzburg D, von Elert E (2004) Impact of 10 dietary sterols on growth and reproduction of Daphnia galeata. J Chem Ecol 30:483–500
Martin-Creuzburg D, von Elert E (2009) Ecological significance of sterols in aquatic food webs. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 43–64
Martin-Creuzburg D, von Elert E, Hoffmann KH (2008) Nutritional constraints at the cyanobacteria-Daphnia magna interface: The role of sterols. Limnol Oceanogr 53:456–468
Martin-Creuzburg D, Wacker A, Basen T (2010) Interactions between limiting nutrients: Consequences for somatic and population growth of Daphnia magna. Limnol Oceanogr 55:2597–2607
Martin-Creuzburg D, Wacker A, von Elert E (2005b) Life history consequences of sterol availability in the aquatic keystone species Daphnia. Oecologia 144:362–372
Martin-Creuzburg D, Wacker A, Ziese C, Kainz MJ (2012) Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168:901–912
Moreau RA, Whitaker BD, Hicks KB (2002) Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41:457–500
Nasir H, Noda H (2003) Yeast-like symbiotes as a sterol source in anobiid beetles (Coleoptera, Anobiidae): Possible metabolic pathways from fungal sterols to 7-dehydrocholesterol. Arch Insect Biochem Physiol 52:175–182
Ohvo-Rekilä H, Ramstedt B, Leppimäki P, Slotte JP (2002) Cholesterol interactions with phospholipids in membranes. Prog Lipid Res 41:66–97
Piepho HP (2004) An algorithm for a letter-based representation of all-pairwise comparisons. J Comput Graph Stat 13:456–466
Piepho M, Martin-Creuzburg D, Wacker A (2010) Simultaneous effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PLoS ONE 5:e15828
Porter JA, Young KE, Beachy PA (1996) Cholesterol modification of hedgehog signaling proteins in animal development. Science 274:255–259
Prahl FG, Eglinton G, Corner EDS, O’Hara SCM, Forsberg TEV (1984) Changes in plant lipids during passage through the gut of Calanus. J Mar Biol Assoc UK 64:317–334
Sakurai S, Yonemura N, Fujimoto Y, Hata F, Ikekawa N (1986) 7-dehydrosterols in prothoracic glands of the silkworm, Bombyx mori. Experientia 42:1034–1036
Sperfeld E, Martin-Creuzburg D, Wacker A (2012) Multiple resource limitation theory applied to herbivorous consumers: Liebig’s minimum rule vs. interactive co-limitation. Ecol Lett 15:142–150
Sperfeld E, Wacker A (2009) Effects of temperature and dietary sterol availability on growth and cholesterol allocation of the aquatic keystone species Daphnia. J Exp Biol 212:3051–3059
Sperfeld E, Wacker A (2011) Temperature- and cholesterol-induced changes in eicosapentaenoic acid limitation of Daphnia magna determined by a promising method to estimate growth saturation thresholds. Limnol Oceanogr 56:1273–1284
Svoboda JA (1999) Variability of metabolism and function of sterols in insects. Crit Rev Biochem Mol 34:49–57
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Volkman JK (2003) Sterols in microorganisms. Appl Microbiol Biot 60:495–506
Wacker A, Martin-Creuzburg D (2012) Biochemical nutrient requirements of the rotifer Brachionus calyciflorus: co-limitation by sterols and amino acids. Funct Ecol 26:1135–1143
Acknowledgments
We thank P. Merkel for technical assistance in analyzing sterols. DMC was supported financially by the ‘Young Scholar Fund’ of the University of Konstanz, AW was supported by the German Research Foundation (DFG, WA 2,445/8-1).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 107 kb)
Rights and permissions
About this article
Cite this article
Martin-Creuzburg, D., Oexle, S. & Wacker, A. Thresholds for Sterol-Limited Growth of Daphnia magna: A Comparative Approach Using 10 Different Sterols. J Chem Ecol 40, 1039–1050 (2014). https://doi.org/10.1007/s10886-014-0486-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0486-1