Abstract
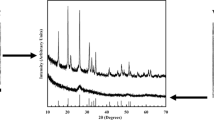
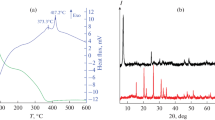
Reaction of VO(OiPr)3/citric acid premixes with excess water produces stable, blue dispersions of VxOy gel nanoparticles (5–100 nm in diameter) that can be isolated via acetone precipitation. Annealing under reducing conditions transforms these gel particles into crystalline, faceted VO2 nanoparticles of similar size. Larger VxOy gel particles (75–200 nm in diameter) form when VxOy nanogel dispersions are aged with aqueous ammonia. Upon annealing, these larger gel particles transform into crystalline VO2 rods of 50 nm–10 μm in length. Hysteresis loops confirming a semiconductor-to-metal phase transition near 68 °C expected for crystalline VO2 particles are recorded by variable-temperature electrical resistance and powder X-ray diffraction measurements.








Similar content being viewed by others
References
M. Imada, A. Fujimori, and Y. Tokura (1998). Rev. Mod. Phys. 70, 1039.
S.-Y. Li, G. A. Niklasson, and C. G. Granqvist (2012). Thin Solid Films 520, 3823.
K. C. Kam and A. K. Cheetham (2006). Mater. Res. Bull. 41, 1015.
T. D. Manning, I. P. Parkin, M. E. Pemble, D. Sheel, and D. Vernardou (2004). Chem. Mater. 16, 744.
S. M. Babulanam, T. S. Eriksson, G. A. Niklasson, and C. G. Granqvist (1987). Solar Energy Mater. 16, 347.
X. Chen, F. Wang, and J. Xu (2011). Top. Catal. 54, 1016.
S. Song, S. Jiang, R. Rao, H. Yang, and A. Zhang (2011). Appl. Catal. A. 401, 215.
J. Nag and R. F. Haglund Jr (2008). J. Phys. 20, 264016.
I. S. Kim and L. J. Lauhon (2012). Cryst. Growth Des. 12, 1383.
R. Teghil, L. D’Alessio, A. De Bonis, A. Galasso, N. Ibris, A. M. Salvi, A. Santagata, and P. Villani (2009). J. Phys. Chem. A 113, 14969.
S. A. Pauli, R. Herger, P. R. Willmott, E. U. Donev, J. Y. Suh, and R. F. Haglund Jr (2007). J. Appl. Phys. 102, 073527.
H. Miyazaki, Y. Iiguni, Y. Tanaka, H. Suzuki, and T. Ota (2013). J. Ceram. Soc. Jpn. 121, 100.
X. Xu, X. He, H. Wang, Q. Gu, S. Shi, H. Xing, C. Wang, J. Zhang, X. Chen, and J. Chu (2012). Appl. Surf. Sci. 261, 83.
F. Wang, Y. Liu, and C.-Y. Liu (2009). J. Solid State Chem. 182, 3249.
Z. Peng, Y. Wang, Y. Du, D. Lu, and D. Sun (2009). J. Alloys Compds. 480, 537.
Z. Peng, W. Jiang, and H. Liu (2007). J. Phys. Chem. C 111, 1119.
S. Ding, Z. Liu, D. Li, W. Zhao, Y. Wang, D. Wan, and F. Huang (2013). ACS Appl. Mater. Interfaces 5, 1630.
J. Zhou, Y. Gao, X. Liu, Z. Chen, L. Dai, C. Cao, H. Luo, M. Kanahira, C. Sun, and L. Yan (2013). Phys. Chem. Chem. Phys. 15, 7505.
Y. Gao, C. Cao, L. Dai, H. Luo, M. Kanehira, Y. Ding, and Z. L. Wang (2012). Energy Environ. Sci. 5, 8708.
S. Ji, F. Zhang, and P. Jin (2011). Solar Energy Mater. Solar Cells 95, 3520.
B. Li, X. Ni, F. Zhou, J. Cheng, H. Zheng, and M. Ji (2006). Solid State Sci. 8, 1168.
S. A. Johnson, P. J. Olliver, and T. E. Mallouk (1999). Science 283, 963.
Z. Lu, C. Li, and Y. Yin (2011). J. Mater. Chem. 21, 14776.
J. M. Booth and P. S. Casey (2009). ACS Appl. Mater. Interfaces 9, 1899.
L. M. Sullivan, L. Li, and C. M. Lukehart (2013). J. Clust. Sci. doi:10.1007/s10876-013-0665-1.
C. Lukehart, L. M. Sullivan, L. Li, and W. H. Morris, III (2012). US Patent 8318128 B2.
J.-H. Son, J. Wei, D. Cobden, G. Cao, and Y. Xia (2010). Chem. Mater. 22, 3043.
A. Gentle, A. I. Maaroof, and G. B. Smith (2007). Nanotechnology 18, 025202.
F. Guinneton, L. Sauques, J. C. Valmalette, F. Cros, and J. R. Gavarri (2001). J. Phys. Chem. Solids 62, 1229.
J. M. Longo and P. Kierkegaard (1970). Acta Chem. Scand. 24, 420.
D. B. McWhan, M. Marezio, J. P. Remeika, and P. D. Dernier (1974). Phys. Rev. B 10, 490.
Acknowledgments
Financial support from the National Science Foundation (No. DMR-0210785) and an industrial sponsor is gratefully acknowledged by C.M.L. We also thank the Vanderbilt University Institute for Integrative Biosystems Research and Education for fabrication of the IDA micro-electrode used in this study. A.G.H. acknowledges support by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 0909667. Partial support by the National Science Foundation Tennessee Solar Conversion and Storage using Outreach, Research and Education (TN-SCORE) under NSF EPS 1004083 is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, W.H., Harris, A.G. & Lukehart, C.M. Synthesis of VO2 Nanopowders. Part II. Hydrolysis of Vanadium Alkoxide/Citric Acid Premixes: VO2 Nanostructures of Controlled Shape. J Clust Sci 25, 323–334 (2014). https://doi.org/10.1007/s10876-013-0670-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0670-4