Abstract
Objective
Dynamic cytokine profiles from endogenously activated T cells in transit from lymph node to the infected sites via the blood compartment after recent exposure to Mycobacterium tuberculosis may differentiate disease progressors from non-disease progressors in a BCG-vaccinated population.
Methods
Household contacts (N = 107) from families with (six families) or without (14 families) secondary cases were assessed for Types 1 and 2 cytokines serially in plasma of whole blood cultures without exogenous stimulation. “ARMS” PCR was carried out for detection of single nucleotide polymorphism T/A in IFN-γ +874.
Results
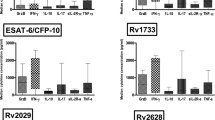
In the absence of IFN-γ expansion, raised IL-4 at 6 months was associated with disease progression in TB-susceptible families. Resistant families on the other hand showed overrepresentation of IFN-γ +874 A allele and expansion of IFN-γ secreting cells at 6 months followed by contraction at 12 months.
Conclusion
Six months may be an important checkpoint for biomarker assessment in high-risk individuals post-exposure.




Similar content being viewed by others
Abbreviations
- CBA:
-
Cytometric bead array
- TB:
-
Tuberculosis
- HC:
-
Household contacts
- PTB-I:
-
Pulmonary TB index case
- AFB:
-
Acid-fast bacilli
- PMN:
-
Pulmonary minimal disease
- PMD:
-
Pulmonary moderate disease
- PAD:
-
Pulmonary advance disease
- DHC:
-
Disease household contacts
- FHC:
-
Household contacts of family with secondary cases
- TST:
-
Tuberculin skin test
- SNP:
-
Single nucleotide polymorphism
- PCR:
-
Polymerase chain reaction
- Tcm:
-
T central memory
- Tem:
-
T effector memory
References
Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An Essential role for Interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54.
Jouanguy E, Dupuis S, Pallier A, Doffinger R, Fondaneche M-D, Fieschi C, et al. In a novel form of INF-γ receptor 1 deficiency, cell surface receptors fail to bind IFN-γ. J Clin Invest. 2000;105(10):1429–36.
Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon gamma receptor deficiency in an infant with fatal Bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–61.
Pierre-Audigier C, Jouanguy E, Lamhamedi S, Atlare F, Rauzier J, Vincent V. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin Infect Dis. 1997;24:982–4.
Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet. 2003;361:1871–2.
Leandro AC, Rocha MA, Cardoso CS, Bonecini-Almeida MG. Genetic polymorphisms in vitamin D receptor, vitamin D-binding protein, Toll-like receptor 2, nitric oxide synthase 2, and interferon-gamma genes and its association with susceptibility to tuberculosis. Braz J Med Biol Res. 2009;42(4):312–22.
Mansouri D, Adimi P, Mirsaeidi M, Mansouri N, Khalilzadeh S, Masjedi MR, et al. Inherited disorders of the IL-12-IFN-gamma axis in patients with disseminated BCG infection. Eur J Pediatr. 2005;164(12):753–7.
Chapgier A, Boisson-Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka-Chalufour A, et al. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease 4. PLoS Genet. 2006;2(8):e131.
Cooke GS, Campbell SJ, Sillah J, Gustafson P, Bah B, Sirugo G, et al. Polymorphism within the Interferon-γ/Receptor Complex is associated with Pulmonary Tuberculosis. Am J Respir Crit Care Med. 2006;174:339–43.
Pacheco AG, Cardoso CC, Moraes MO. IFNG +874T/A, IL10–1082G/A and TNF -308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet. 2008;123(5):477–84.
Nemeth J, Winkler HM, Boeck L, Adegnika AA, Clement E, Mve TM, et al. Specific cytokine patterns of pulmonary tuberculosis in Central Africa. Clin Immunol. 2011;138(1):50–9.
O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10 producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114(10):1372–8.
Dlugovitzky D, Torres-Morales A, Rateni L, Farroni MA, Largacha C, Molteni O, et al. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunol Med Microbiol. 1997;18:203–7.
Verbon A, Juffermans N, Deventer SJH, Speelman P, Deutekom HV, Poll TVD. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110–3.
Hasan Z, Jamil B, Khan J, Ali R, Khan MA, Nasir N, et al. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand J Immunol. 2009;69(3):259–67.
Dlugovitzky D, Bay ML, Rateni L, Urizar L, Rondelli CF, Largacha C, et al. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;49(2):210–7.
Hussain R, Kaleem A, Shahid F, Dojki M, Jamil B, Mehmood H, et al. Cytokine profiles using whole blood assays can discriminate between tuberculosis patients and healthy endemic controls in BCG-vaccinated population. J Immunol Meth. 2002;264:95–108.
Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61(8):3482–8.
Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, et al. Ex Vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis. 2005;40:184–7.
Hirsch CS, Toossi Z, Johnson JL, Luzze H, Ntambi L, Peters P, et al. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis. 2001;183(5):779–88.
Vankayalapati R, Wizel B, Weis SE, Klucar P, Shams H, Samten B, et al. Serum cytokine concentrations do not parallel Mycobacterium tuberculosis-induced cytokine production in patients with tuberculosis. Clin Infect Dis. 2003;36:24–8.
Jason J, Archibald LK, Nwanyanwu OC, Byrd MG, Kazembe PN, Dobbie H, et al. Comparison of serum and cell-specific cytokines in humans. Clin Diag Lab Immunol. 2001;8(6):1097–103.
WHO. Global Tuberculosis Control WHO report 2009: WHO/HTM/TB/2009411. Geneva: WHO; 2009. Report No.: WHO report 2009 Country Profile.
Akhtar S, White F, Hasan R, Rozi S, Younus M, Ahmed F, et al. Hyperendemic pulmonary tuberculosis in peri-urban areas of Karachi, Pakistan. BMC Public Health. 2007;7:70.
Tapaninen P, Korhonen A, Pusa L, Seppala I, Tuuminen T. Effector memory T-cells dominate immune responses in tuberculosis treatment: antigen or bacteria persistence? Int J Tuberc Lung Dis. 2010;14(3):347–55.
Talat N, Shahid F, Perry S, Dawood G, Hussain R. Th1/Th2 Cytometric Bead Array can discriminate cytokine secretion from endogenously activated cells in pulmonary disease, recent and remote infection in tuberculosis. Cytokine. 2011;54(2):136–43.
Crofton J. Crofton and Douglas respiratory diseases. Clinical features of tuberculosis. 4th ed. London: Blackwell; 1990. p. 395–421.
Ansari A, Talat N, Jamil B, Hasan Z, Razzaki T, Dawood G, et al. Cytokine gene polymorphisms across tuberculosis clinical spectrum in Pakistani patients. PLoS One. 2009;4(3):e4778.
Talat N, Shahid F, Dawood G, Hussain R. Dynamic changes in biomarker profiles associated with clinical and subclinical tuberculosis in a high transmission setting: a four-year follow-up study. Scand J Immunol. 2009;69(6):537–46.
Comstock G. Epidemiology of tuberculosis. Am J Respir Crit Care Med. 1982;125:8–15.
Rook GA, Hernandez-Pando R, Dheda K, Teng SG. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25(9):483–8.
Moller M, Hoal EG. Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberculosis (Edinb). 2010;90(2):71–83.
van der Eijk EA, van de Vosse E, Vandenbroucke JP, van Dissel JT. Heredity versus environment in tuberculosis in twins: the 1950s United Kingdom Prophit Survey Simonds and Comstock revisited. Am J Respir Crit Care Med. 2007;176(12):1281–8.
Mendez A, Hernandez-Pando R, Contreras S, Aguilar D, Rook GA. CCL2, CCL18 and sIL-4R in renal, meningeal and pulmonary TB; a 2 year study of patients and contacts. Tuberculosis (Edinb). 2011;91(2):140–5.
Hussain R, Talat N, Shahid F, Dawood G. Longitudinal tracking of cytokines after acute exposure to Tuberculosis: Association of Distinct Cytokine patterns with protection and disease development. Clin Vaccine Immunol. 2007;14(12):1578–86.
Goletti D, Butera O, Bizzoni F, Casetti R, Girardi E, Poccia F. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J Infect Dis. 2006;194:984–92.
Kim SH, Lee CE. Counter-regulation mechanism of IL-4 and IFN-alpha signal transduction through cytosolic retention of the pY-STAT6:pY-STAT2:p48 complex. Eur J Immunol. 2011;41(2):461–72.
Apte SH, Groves P, Olver S, Baz A, Doolan DL, Kelso A, et al. IFN-gamma inhibits IL-4-induced type 2 cytokine expression by CD8 T cells in vivo and modulates the anti-tumor response. J Immunol. 2010;185(2):998–1004.
Bogdan C, Vodovotz Y, Paik J, Xie QW, Nathan C. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol. 1994;55(2):227–33.
Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, et al. Activation and regulation of Toll like receptors 2 and 1 in human leprosy. Nature Med. 2003;9(5):525–32.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35.
Fletcher HA, Owiafe P, Jeffries D, Hill P, Rook GA, Zumla A, et al. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology. 2004;112(4):669–73.
Demissie A, Abebe M, Aseffa A, Rook G, Fletcher H, Zumla A, et al. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J Immunol. 2004;172(11):6938–43.
Luzina IG, Lockatell V, Todd NW, Keegan AD, Hasday JD, Atamas SP. Splice isoforms of human interleukin-4 are functionally active in mice in vivo. Immunology. 2011;132(3):385–93.
Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, et al. In vivo and in vitro studies of a novel cytokine, interleukin 4delta2, in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172(4):501–8.
Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115(2):313–25.
Biselli R, Mariotti S, Sargentini V, Sauzullo I, Lastilla M, Mengoni F, et al. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin Microbiol Infect. 2010;16(8):1282–4.
Sargentini V, Mariotti S, Carrara S, Gagliardi MC, Teloni R, Goletti D, et al. Cytometric detection of antigen-specific IFN-gamma/IL-2 secreting cells in the diagnosis of tuberculosis. BMC Infect Dis. 2009;9:99.
Ribeiro-Rodrigues R. Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, et al. A role of CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34.
Elliot AM, Hodsdon WS, Kyosiimire J, Quigley MA, Nakiyingi JS, Namujju PB, et al. Cytokine responses and progression to active tuberculosis in HIV-1-infected Ugandans: a prospective study. Trans Roy SocTrop Med & Hyg. 2004;98:660–70.
Acknowledgements
We acknowledge the Higher Education Commission (HEC), Government of Pakistan for providing financial support for this project through Grant No. 20-796/R&D/07. We also thank Mr. Mohammed Anwar for blood collection and Ms Muniba Islam for technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Cumulative frequency of disease progression in household contacts (HC = 110) of 20 families. Disease progression occurred over a period of 6–60 months. Diagnosis was based on signs and symptoms, hematology (N = 10), radiology (N = 10), microscopy (N = 3), and response to ATT treatment. Pulmonary disease was diagnosed in 9/10 DHC (4 = minimal, three moderate and two advanced lung involvement) and abdominal in 1/10 DHC. ATT was started immediately at diagnosis. All patients showed complete clinical recovery after ATT. (JPEG 56 kb)
Figure S2
IL-4 and IFN-γ responses in tuberculosis disease susceptible and resistant groups at 6 months post-exposure. Groups were assessed at 6 months (HC = 55, FHC = 30, DHC = 10); results are shown as mean responses (picograms per milliliter). All other details are the same as Fig. 1. (JPEG 38 kb)
Rights and permissions
About this article
Cite this article
Hussain, R., Talat, N., Ansari, A. et al. Endogenously Activated Interleukin-4 Differentiates Disease Progressors and Non-Progressors in Tuberculosis Susceptible Families: A 2-Year Biomarkers Follow-Up Study. J Clin Immunol 31, 913–923 (2011). https://doi.org/10.1007/s10875-011-9566-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9566-y