Abstract
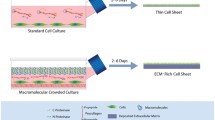
In this study we applied a smart biomaterial formed from a self-assembling, multi-functional synthetic peptide amphiphile (PA) to coat substrates with various surface chemistries. The combination of PA coating and alignment-inducing functionalised substrates provided a template to instruct human corneal stromal fibroblasts to adhere, become aligned and then bio-fabricate a highly-ordered, multi-layered, three-dimensional tissue by depositing an aligned, native-like extracellular matrix. The newly-formed corneal tissue equivalent was subsequently able to eliminate the adhesive properties of the template and govern its own complete release via the action of endogenous proteases. Tissues recovered through this method were structurally stable, easily handled, and carrier-free. Furthermore, topographical and mechanical analysis by atomic force microscopy showed that tissue equivalents formed on the alignment-inducing PA template had highly-ordered, compact collagen deposition, with a two-fold higher elastic modulus compared to the less compact tissues produced on the non-alignment template, the PA-coated glass. We suggest that this technology represents a new paradigm in tissue engineering and regenerative medicine, whereby all processes for the bio-fabrication and subsequent self-release of natural, bio-prosthetic human tissues depend solely on simple template-tissue feedback interactions.






Similar content being viewed by others
References
Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Advanced Healthc Mater. 2013;2:57–71.
Jones RR, Hamley IW, Connon CJ. Chapter 4 the instructive role of biomaterials in cell-based therapy and tissue engineering. Hydrogels in cell-based therapies. Cambridge: The Royal Society of Chemistry; 2014. p. 73–94.
Liu JS, Gartner ZJ. Directing the assembly of spatially organized multicomponent tissues from the bottom up. Trends Cell Biol. 2012;22:683–91.
Samchenko Y, Ulberg Z, Korotych O. Multipurpose smart hydrogel systems. Adv Colloid Interface Sci. 2011;168:247–62.
Hayashida Y, Nishida K, Yamato M, Yang J, Sugiyama H, Watanabe K, et al. Transplantation of tissue-engineered epithelial cell sheets after excimer laser photoablation reduces postoperative corneal haze. Invest Ophthalmol Vis Sci. 2006;47:552–7.
Dworak A, Utrata-Wesolek A, Oleszko N, Walach W, Trzebicka B, Aniol J, et al. Poly(2-substituted-2-oxazoline) surfaces for dermal fibroblasts adhesion and detachment. J Mater Sci Mater Med. 2014;25:1149–63.
Takagi R, Murakami D, Kondo M, Ohki T, Sasaki R, Mizutani M, et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253–9.
Hama T, Yamamoto K, Yaguchi Y, Murakami D, Sasaki H, Yamato M, et al. Autologous human nasal epithelial cell sheet using temperature-responsive culture insert for transplantation after middle ear surgery. J Tissue Eng Regen Med. 2015. doi:10.1002/term.2012.
Sakaguchi K, Shimizu T, Okano T. Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release. 2015;205:83–8.
Aranaz I, Martinez-Campos E, Nash ME, Tardajos MG, Reinecke H, Elvira C, et al. Pseudo-double network hydrogels with unique properties as supports for cell manipulation. J Mater Chem B. 2014;2:3839–48.
Hruschka V, Saeed A, Slezak P, Cheikh Al Ghanami R, Feichtinger GA, Alexander C, et al. Evaluation of a thermoresponsive polycaprolactone scaffold for in vitro three-dimensional stem cell differentiation. Tissue Eng Part A. 2015;21:310–9.
Sekiya S, Shimizu T, Okano T. Vascularization in 3D tissue using cell sheet technology. Regen Med. 2013;8:371–7.
Matsuura K, Utoh R, Nagase K, Okano T. Cell sheet approach for tissue engineering and regenerative medicine. J Control Release. 2014;190:228–39.
Boekhoven J, Stupp SI. 25th Anniversary article: supramolecular materials for regenerative medicine. Adv Mater. 2014;26:1642–59.
Hamley IW. Lipopeptides: from self-assembly to bioactivity. Chem Commun. 2015;51:8574–83.
Dehsorkhi A, Gouveia RM, Smith AM, Hamley IW, Castelletto V, Connon CJ, et al. Self-assembly of a dual functional bioactive peptide amphiphile incorporating both matrix metalloprotease substrate and cell adhesion motifs. Soft Matter. 2015;11:3115–24.
Gouveia RM, Castelletto V, Hamley IW, Connon CJ. New self-assembling multifunctional templates for the biofabrication and controlled self-release of cultured tissue. Tissue Eng Part A. 2015;21:1772–84.
Miotto M, Gouveia RM, Connon CJ. Peptide amphiphiles in corneal tissue engineering. J Funct Biomater. 2015;6:687–707.
Lewis PN, Pinali C, Young RD, Meek KM, Quantock AJ, Knupp C. Structural interactions between collagen and proteoglycans are elucidated by three-dimensional electron tomography of bovine cornea. Structure. 2010;18:239–45.
Gouveia RM, Castelletto V, Alcock SG, Hamley IW, Connon CJ. Bioactive films produced from self-assembling peptide amphiphiles as versatile substrates for tuning cell adhesion and tissue architecture in serum-free conditions. J Mater Chem B. 2013;1:6157–69.
Gouveia RM, Connon CJ. The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions. Invest Ophthalmol Vis Sci. 2013;54:7483–91.
Wittmann JC, Smith P. Highly oriented thin-films of poly(tetrafluoroethylene) as a substrate for oriented growth of materials. Nature. 1991;352:414–7.
Lin DC, Horkay F. Nanomechanics of polymer gels and biological tissues: a critical review of analytical approaches in the Hertzian regime and beyond. Soft Matter. 2008;4:669–82.
Holmes DF, Gilpin CJ, Baldock C, Ziese U, Koster AJ, Kadler KE. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci USA. 2001;98:7307–12.
Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–58.
Chen B, Jones RR, Mi SL, Foster J, Alcock SG, Hamley IW, et al. The mechanical properties of amniotic membrane influence its effect as a biomaterial for ocular surface repair. Soft Matter. 2012;8:8379–87.
Hamley IW, Castelletto V, Moulton CM, Rodriguez-Perez J, Squires AM, Eralp T, et al. Alignment of a model amyloid peptide fragment in bulk and at a solid surface. J Phys Chem B. 2010;114:8244–54.
Foster JW, Jones RR, Bippes CA, Gouveia RM, Connon CJ. Differential nuclear expression of Yap in basal epithelial cells across the cornea and substrates of differing stiffness. Exp Eye Res. 2014;127:37–41.
Loparic M, Wirz D, Daniels AU, Raiteri R, Vanlandingham MR, Guex G, et al. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite. Biophys J. 2010;98:2731–40.
Stolz M, Raiteri R, Daniels AU, VanLandingham MR, Baschong W, Aebi U. Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys J. 2004;86:3269–83.
Achterberg VF, Buscemi L, Diekmann H, Smith-Clerc J, Schwengler H, Meister JJ, et al. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J Invest Dermatol. 2014;134:1862–72.
Crichton ML, Donose BC, Chen X, Raphael AP, Huang H, Kendall MA. The viscoelastic, hyperelastic and scale dependent behaviour of freshly excised individual skin layers. Biomaterials. 2011;32:4670–81.
Lombardo M, Lombardo G, Carbone G, De Santo MP, Barberi R, Serrao S. Biomechanics of the anterior human corneal tissue investigated with atomic force microscopy. Invest Ophthalmol Vis Sci. 2012;53:1050–7.
Shen ZL, Kahn H, Ballarini R, Eppell SJ. Viscoelastic properties of isolated collagen fibrils. Biophys J. 2011;100:3008–15.
Acknowledgments
We thank Dr C. A. Bipes from Nanosurf AG, Switzerland for his expert technical support in atomic force microscopy. This work was funded by the Biotechnology and Biological Sciences Research Council (BBSRC Grant Reference BB/I008187/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gouveia, R.M., Hamley, I.W. & Connon, C.J. Bio-fabrication and physiological self-release of tissue equivalents using smart peptide amphiphile templates. J Mater Sci: Mater Med 26, 242 (2015). https://doi.org/10.1007/s10856-015-5581-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5581-5