Abstract
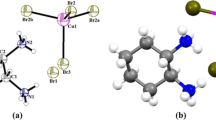
A comprehensive theoretical density functional theory (DFT) study of the electronic crystal structure, bonding properties, electron charge density of C11H8N2O o-methoxydicyanovinylbenzene (DIVA) single crystals were performed. The exchange and correlation potential was described within a framework of the local density approximation (LDA) by Ceperley-Alder and gradient approximation (GGA) based on exchange–correlation energy optimization to calculate the total energy. In addition, we have used Engel–Vosko generalized gradient approximation (EV-GGA) and the modified Becke–Johnson potential (mBJ) for the electronic crystal structure, bonding properties, electron charge density calculations. There is systematically increasing in the energy gap from 2.25 eV (LDA), 2.34 eV (GGA), 2.50 eV (EV-GGA), 2.96 eV (mBJ). Our calculations show that this crystal possess direct energy gap. Furthermore, the electronic charge density space distribution contours in the (1 1 0) crystallographic plane clarifies the nature of chemical bonding.




Similar content being viewed by others
References
Zyss J., Ledoux I., Nicoud J. F. (1994). Mole
Desiraju GR (2002) Acc Chem Res 35:565
Aakeroy CB, Seddon KR (1993) Chem Soc Rev 22:397
Saha BK, Nangia A, Jaskolski M (2005) Cryst Eng Comm 7:355
Russell VA, Etter MC, Ward MD (1994) J Am Chem Soc 116:1941
Huang KS, Britton D, Etter MC, Byrn SR (1995) J Mater Chem 5:379
Panunto TW, Urbanczyk-Lipkowska Z, Johnson R, Etter MC (1987) J Am Chem Soc 109:7786
R. Custelcean, Chem. Commun. (Cambridge) 2008, 295
Yin Z, Li Z (2006) Tetrahedron Lett 47:7875
Jazbinsek M, Kwon OP, Bosshard Ch, Günter P (2008) handbook of organic electronics and photonics. In: Nalwa SH (ed), American Scientific Publishers, Los Angeles
Bosshard Ch, Bösch M, Liakatas I, Jäger M, Günter P (2000) Nonlinear optical effects and materials. In: Günter P (ed), Springer, Berlin
Nalwa HS, Watanabe T, Miyata S (1997) Nonlinear optics of organic molecules and polymers. In: Nalwa HS, Miyata S (eds), CRC, Boca Raton
Zyss J, Oudar JL (1982) Phys Rev A 26:2028
Kwon O-P, Jazbinsek M, Seo J-I, Choi E-Y, Yun H, Fabian DJ, Brunner Y, Lee S, Günter P (2009) J Chem Phys 130:134708
Koch W, Holthausen MCAA (2000) Chemistry guide to density functional theory. Wiley, Weinheim
Parr RR, Yang RG (1989) Density functional theory of atoms and molecules. Oxford University Press, New York and references therein
Gao S (2003) Comput Phys Commun 153:190
Schwarz K (2003) J Solid State Chem 176:319
Antipin MY, Barr AT, Cardelino HB, Clark DR, Moore EC, Myers T, Penn B, Romero M, Timofeeva VMST (1997) J Phys Chem B 101:2770
Blaha P, Schwarz K, Madsen GKH, Kvasnicka D, Luitz J (2001) WIEN2 K, an augmented plane wave + local orbitals program for calculating crystal properties, Karlheinz Schwarz. Techn Universitat Wien, Wien. ISBN 3-9501031-1-2
Hohenberg P, Kohn W (1964) Phys Rev B 136:864
Ceperley DM, Ader BI (1980) Phys Rev Lett 45:566
Perdew JP, Zunger A (1973) Phys Rev B 8:4822
Perdew JP, Burke S, Ernzerhof M (1996) Phys Rev Lett 77:3865
Engel E, Vosko SH (1993) Phys Rev B 47:13164
Tran F, Blaha P (2009) Phys Rev Lett 102:226401
Acknowledgements
This study was supported from the institutional research concept of the project CENAKVA (No. CZ.1.05/2.1.00/01.0024), the grant No. 152/2010/Z of the Grant Agency of the University of South Bohemia. The School of Materials Engineering, University Malaysia Perlis (UniMAP), Perlis, Malaysia. S.A. thanks Council of Scientific and Industrial Research (CSIR) - National Physical Laboratory for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reshak, A.H., Kamarudin, H., Kityk, I.V. et al. Electronic structure, charge density, and chemical bonding properties of C11H8N2O o-methoxydicyanovinylbenzene (DIVA) single crystal. J Mater Sci 48, 5157–5162 (2013). https://doi.org/10.1007/s10853-013-7301-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7301-1