Abstract
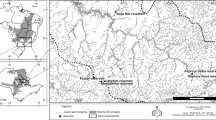
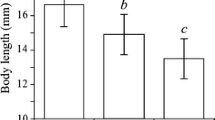
The principle of limiting similarity states that closely related species need to partition resources of the habitat in order to coexist in the same general area. We tested this hypothesis experimentally with a sister species pair of non-biting midges (Chironomus riparius and C. piger) by assessing their relative larval fitness under several concentrations of nitrite and temperature regimes, as suggested by the observed habitat segregation in a previous field study. Both chironomid species often occur in eutrophic habitats like agricultural areas or industrial point source effluents. Based on field observations, we hypothesised C. piger to tolerate higher nitrite concentrations, higher temperatures and larger temperature ranges than C. riparius. As predicted, C. piger coped better with higher nitrite concentrations. Against the expectations, C. riparius had a tendentially higher fitness at both higher constant temperatures and larger daily temperature ranges. However, the interaction of both stressors favoured C. piger in warm high-nitrite habitats thus concurring to the field observations. The complex interaction of candidate environmental factors with antagonistic effects found here emphasises thus the necessity to experimentally assess field observations of niche segregation.



Similar content being viewed by others
References
Albrecht, M. & N. J. Gotelli, 2001. Spatial and temporal niche partitioning in grassland ants. Oecologia 126: 134–141.
Alonso, A. & J. A. Camargo, 2003. Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bulletin of Environmental Contamination and Toxicology 70: 1006–1012.
Amarasekare, P., 2003. Competitive coexistence in spatially structured environments: a synthesis. Ecology Letters 6: 1109–1122.
Armitage, P. D., P. S. Cranston & L. C. V. Pinder, 1995. The chironomidae: the biology and ecology of non-biting midges. Chapman & Hall, London.
Arthur, W., 1987. The niche in competition and evolution. Wiley, New York.
Atkins, P. & J. De Paula, 2006. Physical chemistry. Oxford University Press, New York.
Attrill, M. J. & M. Power, 2004. Partitioning of temperature resources amongst an estuarine fish assemblage. Estuarine Coastal and Shelf Science 61: 725–738.
Bechard, K. M., P. L. Gillis & C. M. Wood, 2008. Acute toxicity of waterborne Cd, Cu, Pb, Ni, and Zn to first-instar Chironomus riparius larvae. Archives of Environmental Contamination and Toxicology 54: 454–459.
Braun, V., R. R. Crichton & G. Braunitz, 1968. Hemoglobins.15. Monomeric and dimeric insect hemoglobins (Chironomus thummi). Hoppe-Seylers Zeitschrift Fur Physiologische Chemie 349: 197–210.
Danks, H. V., 1978. Some effects of photoperiod, temperature, and food on emergence in 3 species of Chironomidae (Diptera). Canadian Entomologist 110: 289–300.
Darwin, C., 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London.
Dietrich, B. & R. Wehner, 2003. Sympatry and allopatry in two desert ant sister species: how do Cataglyphis bicolor and C-savignyi coexist? Oecologia 136: 63–72.
Geervliet, J. B. F., M. S. W. Verdel, H. Snellen, J. Schaub, M. Dicke & L. E. M. Vet, 2000. Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C-rubecula in the Netherlands: predicting field performance from laboratory data. Oecologia 124: 55–63.
Gunderina, L. I., I. I. Kiknadze, A. G. Istomina, V. D. Gusev & L. A. Miroshnichenko, 2005. Divergence of the polytene chromosome banding sequences as a reflection of evolutionary rearrangements of the genome linear structure. Russian Journal of Genetics 41: 130–137.
Guryev, V., I. Makarevitch, A. Blinov & J. Martin, 2001. Phylogeny of the Genus Chironomus (Diptera) inferred from DNA sequences of mitochondrial cytochrome b and cytochrome oxidase I. Molecular Phylogenetics and Evolution 19: 9–21.
Haas, H. S. & K. Strenzke, 1957. Experimentelle untersuchungen über den Einfluß der ionalen Zusammensetzung des mediums auf die entwicklung der analpapillen von Chironomus thummi. Biologisches Zentralblatt 76: 513–528.
Hommen, U., 2005. Ableitung von populationswachstumsraten aus Lebensdatenstudien mit Chironomus riparius. Frauenhofer Institut für Molekularbiologie und angewandte Ökologie, Schmallenberg.
Hutchinson, G. E., 1957. Population studies – animal ecology and demography – concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427.
Kahlert, M. & D. Neumann, 1997. Early development of freshwater sponges under the influence of nitrite and pH. Archiv für Hydrobiologie 139: 69–81.
Kelso, B. H. L., D. M. Glass & R. V. Smith, 1999. Toxicity of nitrite to freshwater invertebrates. In Wilson, W. S., A. S. Ball & R. H. Hinton (eds), Managing risks of nitrates to human and the environ environment. Royal Society of Chemistry, Cambridge: 175–188.
Langeland, A., J. H. Abeelund, B. Jonsson & N. Jonsson, 1991. Resource partitioning and Niche shift in Arctic Charr Salvelinus-alpinus and Brown Trout Salmo-trutta. Journal of Animal Ecology 60: 895–912.
Maier, K. J., P. Kosalwat & A. W. Knight, 1990. Culture of Chironomus decorus (Diptera, Chironomidae) and the effect of temperature on its life-history. Environmental Entomology 19: 1681–1688.
Mayr, E., 1942. Systematics and the origin of species. Dover Publications, New York.
McArthur, R. L. & R. Levins, 1967. The limiting similarity, convergence and divergence of coexisting species. American Naturalist 101: 377–385.
McLachlan, A., 1993. Can 2 species of midge coexist in a single puddle of rainwater. Hydrobiologia 259: 1–8.
Meszena, G., M. Gyllenberg, L. Pasztor & J. A. J. Metz, 2006. Competitive exclusion and limiting similarity: a unified theory. Theoretical Population Biology 69: 68–87.
Nebeker, A. V., M. A. Cairns & C. M. Wise, 1984. Relative sensitivity of Chironomus-tentans life stages to copper. Environmental Toxicology and Chemistry 3: 151–158.
Nemec, S., 2009. Einfluss ausgewählter abiotischer Faktoren auf die relative Fitness der Schwesterarten Chironomus riparius und Chironomus piger. Diploma thesis. Goethe University, Frankfurt am Main.
Neumann, D., M. Kramer, I. Raschke & B. Grafe, 2001. Detrimental effects of nitrite on the development of benthic Chironomus larvae, in relation to their settlement in muddy sediments. Archiv für Hydrobiologie 153: 103–128.
Nowak, C., D. Jost, C. Vogt, M. Oetken, K. Schwenk & J. Oehlmann, 2007a. Consequences of inbreeding and reduced genetic variation on tolerance to cadmium stress in the midge Chironomus riparius. Aquatic Toxicology 85: 278–284.
Nowak, C., C. Vogt, J. B. Diogo & K. Schwenk, 2007b. Genetic impoverishment in laboratory cultures of the test organism Chironomus riparius. Environmental Toxicology and Chemistry 26: 1018–1022.
Nowak, C., C. Vogt, M. Pfenninger, K. Schwenk, J. Oehlmann, B. Streit & M. Oetken, 2009. Rapid genetic erosion in pollutant-exposed experimental chironomid populations. Environmental Pollution 157: 881–886.
OECD. (2004) Sediment-water chironomid toxicity test using spiked water. OECD guidelines for the testing of chemicals (Original guideline 219, adopted 13 Apr 2004).
Oliver, D. R., 1971. Life history of Chironomidae. Annual Review of Entomology 16: 211–230.
Pery, A. R. R. & J. Garric, 2006. Modelling effects of temperature and feeding level on the life cycle of the midge Chironomus riparius: an energy-based modelling approach. Hydrobiologia 553: 59–66.
Pfenninger, M. & C. Nowak, 2008. Reproductive isolation and ecological niche partition among larvae of the morphologically cryptic sister species Chironomus riparius and C. piger. PLoS ONE 3: e2157.
Pfenninger, M., C. Nowak, C. Kley, D. Steinke & B. Streit, 2007. Utility of DNA taxonomy and barcoding for the inference of larval community structure in morphologically cryptic Chironomus (Diptera) species. Molecular Ecology 16: 1957–1968.
Sankarperumal, G. & T. J. Pandian, 1991. Effect of temperature and chlorella density on growth and metamorphosis of Chironomus circumdatus (Kieffer) (Diptera). Aquatic Insects 13: 167–177.
Sher, R. B. & E. J. Shields, 1991. Potato leafhopper (Homoptera, Cicadellidae) oviposition and development under cool fluctuating temperatures. Environmental Entomology 20: 1113–1120.
Sibly, R. M. & J. Hone, 2002. Population growth rate and its determinants: an overview. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 357: 1153–1170.
Stevens, M. M., 1998. Development and survival of Chironomus tepperi Skuse (Diptera: Chironomidae) at a range of constant temperatures. Aquatic Insects 20: 181–188.
Stief, P., D. De Beer & D. Neumann, 2002. Small-scale distribution of interstitial nitrite in freshwater sediment microcosms: the role of nitrate and oxygen availability, and sediment permeability. Microbial Ecology 43: 367–378.
Strenzke, K., 1960. Die systematische und ökologische differenzierung der gattung Chironomus. Annales Entomologici Fennici 26: 111–139.
Svensson, J. M., 1998. Emission of N2O, nitrification and denitrification in a eutrophic lake sediment bioturbated by Chironomus plumosus. Aquatic Microbial Ecology 14: 289–299.
Thienemann, A., 1974. Die Binnengewässer. Band XX: Chironomus. E. Schweizerbart′sche Verlagsbuchhandlung, Stuttgart.
Tokeshi, M., 1995. Randomness and aggregation – analysis of dispersion in an epiphytic Chironomus community. Freshwater Biology 33: 567–578.
Toquenaga, Y. & K. Fujii, 1991. Contest and scramble competitions in 2 Bruchid species, Callosobruchus analis and C-Phaseoli (Coleoptera, Bruchidae).2. Larval competition experiment. Researches on Population Ecology 33: 129–139.
Trewitt, P. M., R. A. Luhm, F. Samad, S. Ramakrishnan, W. Y. Kao & G. Bergtrom, 1995. Molecular evolutionary analysis of the YWVZ/7B Globin gene-cluster of the insect Chironomus thummi. Journal of Molecular Evolution 41: 313–328.
Vogt, C., D. Belz, S. Galluba, C. Nowak, M. Oetken & J. Oehlmann, 2007a. Effects of cadmium and tributyltin on development and reproduction of the non-biting midge Chironomus riparius (Diptera) – baseline experiments for future multi-generation studies. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering 42: 1–9.
Vogt, C., C. Nowak, J. B. Diogo, M. Oetken, K. Schwenk & J. Oehlmann, 2007b. Multi-generation studies with Chironomus riparius – effects of low tributyltin concentrations on life history parameters and genetic diversity. Chemosphere 67: 2192–2200.
Vogt, C., A. Pupp, C. Nowak, L. S. Jagodzinski, J. Baumann, D. Jost, M. Oetken & J. Oehlmann, 2007c. Interaction between genetic diversity and temperature stress on life-cycle parameters and genetic variability in midge Chironomus riparius populations. Climate Research 33: 207–214.
Wiens, J. J. & C. H. Graham, 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual review of ecology evolution and systematics. Annual Review of Ecology Evolution and Systematics 36: 519–539.
Williams, K. A., D. W. J. Green, D. Pascoe & D. E. Gower, 1986. The Acute toxicity of cadmium to different larval stages of Chironomus-riparius (Diptera, Chironomidae) and its ecological significance for pollution regulation. Oecologia 70: 362–366.
Acknowledgments
The kind assistance of Lucas Jagodzinski, Christiane Frosch and the staff of the Departments Aquatic Ecotoxicology and Ecology & Evolution of the Goethe-Universität Frankfurt am Main is greatly appreciated. We also thank Christian Abel and Simit Patel for language corrections. This research project has been funded by the research funding programme “LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of Hesse’s Ministry of Higher Education, Research, and the Arts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Rights and permissions
About this article
Cite this article
Nemec, S., Heß, M., Nowak, C. et al. Experimental evidence for niche segregation in a sister species pair of non-biting midges. Hydrobiologia 691, 203–212 (2012). https://doi.org/10.1007/s10750-012-1074-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1074-4