Abstract
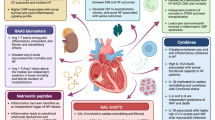
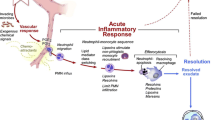
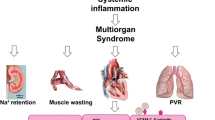
Heart failure (HF) with preserved ejection fraction (HFPEF) is an increasingly prevalent clinical syndrome with many unresolved issues regarding diagnosis, pathophysiology, and treatment. The major pathophysiological mechanisms underlying HFPEF are known to be fibrosis and reduced ventricular compliance, and hypertension (HTN) is perhaps the most significant risk factor for the development of left ventricular diastolic dysfunction (LVDD). Inflammation is one of the earliest events in cardiac stress situations such as pressure and/or volume overload and involves elevated levels of endothelial adhesion molecules as well as increased production and release of inflammatory cytokines and chemokines in the tissue. The latter promotes the infiltration of activated inflammatory cells, particularly monocytes, into the cardiac tissue. Increased monocyte infiltration is seen in the early and late stages of HTN and HFPEF. Once inside the tissue, monocytes differentiate into macrophages and promote cardiac inflammation, tissue injury, and myocardial fibrosis. This review focuses on inflammation as the initial and primary trigger of ventricular remodelling in HTN and LVDD, affecting progression to HFPEF. The link between inflammation and b-type natriuretic peptide (BNP), a clinical marker of cardiac pressure overload which is positively associated with cardiac dysfunction and HF, is also described. Finally, current and prospective therapeutic approaches for HFPEF based on modification of the inflammatory response are reviewed.

Similar content being viewed by others
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14 (8):803–869
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119(14):e391–e479
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259
Hogg K, Swedberg K, McMurray J (2004) Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 43(3):317–327
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodelling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271
Liu Y, Haddad T, Dwivedi G (2013) Heart failure with preserved ejection fraction: current understanding and emerging concepts. Curr Opin Cardiol 28(2):187–196
Badano LP, Albanese MC, De Biaggio P, Rozbowsky P, Miani D, Fresco C, Fioretti PM (2004) Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr 17(3):253–261
Paulus WJ, van Ballegoij JJ (2010) Treatment of heart failure with normal ejection fraction: an inconvenient truth! J Am Coll Cardiol 55(6):526–537
Dubi S, Arbel Y (2010) Large animal models for diastolic dysfunction and diastolic heart failure-a review of the literature. Cardiovasc Pathol 19(3):147–152
McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM (2008) Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail 10(2):149–156
Volpe M, McKelvie R, Drexler H (2010) Hypertension as an underlying factor in heart failure with preserved ejection fraction. J Clin Hypertens (Greenwich) 12(4):277–283
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D (2002) Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 106(24):3068–3072
van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ (2012) Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 9(4):293–302
Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T (2004) Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43(4):739–745
Kai H, Kuwahara F, Tokuda K, Imaizumi T (2005) Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 28(6):483–490
Savoia C, Schiffrin EL (2007) Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 112(7):375–384
Kass DA, Bronzwaer JG, Paulus WJ (2004) What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94(12):1533–1542
van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ (2006) Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113(16):1966–1973
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105(11):1387–1393
Oh JK, Hatle L, Tajik AJ, Little WC (2006) Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 47(3):500–506
Yturralde RF, Gaasch WH (2005) Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis 47(5):314–319
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: part II: causal mechanisms and treatment. Circulation 105(12):1503–1508
Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117(3):568–575
Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP (1989) Patterns of myocardial fibrosis. J Mol Cell Cardiol 21(Suppl 5):121–131
Kuwahara F, Kai H, Tokuda K, Niiyama H, Tahara N, Kusaba K, Takemiya K, Jalalidin A, Koga M, Nagata T, Shibata R, Imaizumi T (2003) Roles of intercellular adhesion molecule-1 in hypertensive cardiac remodelling. Hypertension 41(3 Pt 2):819–823
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4(1):44–52
Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J (2010) Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 55(19):2129–2137
Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM (2011) Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 13(10):1087–1095
Dostal DE, Baker KM (1992) Angiotensin II stimulation of left ventricular hypertrophy in adult rat heart. Mediation by the AT1 receptor. Am J Hypertens 5(5 Pt 1):276–280
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumour necrosis factor in severe chronic heart failure. N Engl J Med 323(4):236–241
Torre-Amione G (2005) Immune activation in chronic heart failure. Am J Cardiol 95(11A):3C–8C; discussion 38C–40C
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91(11):988–998
Adamopoulos S, Parissis JT, Kremastinos DT (2001) A glossary of circulating cytokines in chronic heart failure. Eur J Heart Fail 3(5):517–526
Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93(4):704–711
Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD (1998) Systemic inflammation in patients with heart failure. Eur Heart J 19(5):761–765
Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L (1999) Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 83(3):376–382
Aukrust P, Ueland T, Muller F, Andreassen AK, Nordoy I, Aas H, Kjekshus J, Simonsen S, Froland SS, Gullestad L (1998) Elevated circulating levels of C–C chemokines in patients with congestive heart failure. Circulation 97(12):1136–1143
Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Richards AM, Lam CS (2012) Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 14(12):1338–1347
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359(21):2195–2207
Galkina E, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27:165–197
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795
Hinglais N, Heudes D, Nicoletti A, Mandet C, Laurent M, Bariety J, Michel JB (1994) Colocalization of myocardial fibrosis and inflammatory cells in rats. Lab Invest 70(2):286–294
Nicoletti A, Heudes D, Mandet C, Hinglais N, Bariety J, Michel JB (1996) Inflammatory cells and myocardial fibrosis: spatial and temporal distribution in renovascular hypertensive rats. Cardiovasc Res 32(6):1096–1107
Komatsu S, Panes J, Russell JM, Anderson DC, Muzykantov VR, Miyasaka M, Granger DN (1997) Effects of chronic arterial hypertension on constitutive and induced intercellular adhesion molecule-1 expression in vivo. Hypertension 29(2):683–689
Kohno T, Anzai T, Naito K, Sugano Y, Maekawa Y, Takahashi T, Yoshikawa T, Ogawa S (2008) Angiotensin-receptor blockade reduces border zone myocardial monocyte chemoattractant protein-1 expression and macrophage infiltration in post-infarction ventricular remodelling. Circ J 72(10):1685–1692
Nicoletti A, Michel JB (1999) Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res 41(3):532–543
Vaziri ND (2008) Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis 2(1):1–10
Oghlakian GO, Sipahi I, Fang JC (2011) Treatment of heart failure with preserved ejection fraction: have we been pursuing the wrong paradigm? Mayo Clin Proc Mayo Clin 86(6):531–539
Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J (1997) Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 95(6):1532–1541
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML (2010) Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49(3):499–507
Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML (2007) Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115(5):584–592
Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, Tsuchihashi M, Sugaya T, Charo IF, Kura S, Tsuzuki T, Ishibashi T, Takeshita A, Egashira K (2004) Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res 94(9):1203–1210
Xu J, Lin SC, Chen J, Miao Y, Taffet GE, Entman ML, Wang Y (2011) CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol 301(2):H538–H547
Iyer A, Chan V, Brown L (2010) The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev 6(4):291–297
Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL (2002) Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension 39(2 Pt 2):679–684
Gomez-Garre D, Gonzalez-Rubio ML, Munoz-Pacheco P, Caro-Vadillo A, Aragoncillo P, Fernandez-Cruz A (2010) Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur J Heart Fail 12(9):903–912
Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, De Biase L, Rubattu S, Volpe M (2007) Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 20(7):784–791
Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA (2008) C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: heart and soul study. Eur J Heart Fail 10(1):63–69
Lee KW, Blann AD, Lip GY (2005) Impaired tissue Doppler diastolic function in patients with coronary artery disease: relationship to endothelial damage/dysfunction and platelet activation. Am Heart J 150(4):756–766
Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR (2011) Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem 57(6):874–882
Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H (2011) Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol 57(7):861–869
Souders CA, Bowers SL, Baudino TA (2009) Cardiac fibroblast: the renaissance cell. Circ Res 105(12):1164–1176
Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E (2008) State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 10(9):824–839
de Lemos JA, McGuire DK, Drazner MH (2003) B-type natriuretic peptide in cardiovascular disease. Lancet 362(9380):316–322
van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL (2013) B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 61(14):1498–1506
Kuhn M, Holtwick R, Baba HA, Perriard JC, Schmitz W, Ehler E (2002) Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice. Heart 87(4):368–374
Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A (1995) Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378(6552):65–68
Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94(26):14730–14735
Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O (1998) Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 95(5):2547–2551
Zahabi A, Picard S, Fortin N, Reudelhuber TL, Deschepper CF (2003) Expression of constitutively active guanylate cyclase in cardiomyocytes inhibits the hypertrophic effects of isoproterenol and aortic constriction on mouse hearts. J Biol Chem 278(48):47694–47699
Kishimoto I, Rossi K, Garbers DL (2001) A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci USA 98(5):2703–2706
Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M (2003) Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 111(9):1399–1407
Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M (2000) Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA 97(8):4239–4244
Cao L, Gardner DG (1995) Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 25(2):227–234
Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, Burnett JC Jr (2006) BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol 209(3):943–949
Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC Jr (2002) Brain natriuretic Peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res 91(12):1127–1134
Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA (2004) B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res 94(4):453–461
Watson CJ, Phelan D, Xu M, Collier P, Neary R, Smolenski A, Ledwidge M, McDonald K, Baugh J (2012) Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: a possible defense against transforming growth factor-beta mediated fibrosis. Fibrogenesis Tissue Repair 5(1):9
Vellaichamy E, Khurana ML, Fink J, Pandey KN (2005) Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem 280(19):19230–19242
Vellaichamy E, Kaur K, Pandey KN (2007) Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides 28(4):893–899
Hirota H, Yoshida K, Kishimoto T, Taga T (1995) Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 92(11):4862–4866
Vanderheyden M, Paulus WJ, Voss M, Knuefermann P, Sivasubramanian N, Mann D, Baumgarten G (2005) Myocardial cytokine gene expression is higher in aortic stenosis than in idiopathic dilated cardiomyopathy. Heart 91(7):926–931
de Bold AJ (2009) Cardiac natriuretic peptides gene expression and secretion in inflammation. J Investig Med 57(1):29–32
Haugen E, Chen J, Wikstrom J, Gronros J, Gan LM, Fu LX (2007) Parallel gene expressions of IL-6 and BNP during cardiac hypertrophy complicated with diastolic dysfunction in spontaneously hypertensive rats. Int J Cardiol 115(1):24–28
Giannessi D, Colotti C, Maltinti M, Del Ry S, Prontera C, Turchi S, Labbate A, Neglia D (2007) Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 12(3):265–274
Vaz Perez A, Doehner W, von Haehling S, Schmidt H, Zimmermann AV, Volk HD, Anker SD, Rauchhaus M (2010) The relationship between tumour necrosis factor-alpha, brain natriuretic peptide and atrial natriuretic peptide in patients with chronic heart failure. Int J Cardiol 141(1):39–43
Ahmad S, Otaal PS, Rai TS, Bahl A, Saikia UN, Manoj RK, Thungapathra M, Talwar KK, Khullar M (2009) Circulating proinflammatory cytokines and N-terminal pro-brain natriuretic peptide significantly decrease with recovery of left ventricular function in patients with dilated cardiomyopathy. Mol Cell Biochem 324(1–2):139–145
Ortega O, Gallar P, Munoz M, Rodriguez I, Carreno A, Ortiz M, Molina A, Oliet A, Lozano L, Vigil A (2004) Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract 97(4):c125–c130
Phelan D, Watson C, Martos R, Collier P, Patle A, Donnelly S, Ledwidge M, Baugh J, McDonald K (2012) Modest elevation in BNP in asymptomatic hypertensive patients reflects sub-clinical cardiac remodelling, inflammation and extracellular matrix changes. PLoS ONE 7(11):e49259
Chiurchiu V, Izzi V, D’Aquilio F, Carotenuto F, Di Nardo P, Baldini PM (2008) Brain Natriuretic Peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul Pept 148(1–3):26–32
Shaw SM, Critchley WR, Puchalka CM, Williams SG, Yonan N, Fildes JE (2012) Brain natriuretic peptide induces CD8 + T cell death via a caspase 3 associated pathway–implications following heart transplantation. Transpl Immunol 26(2–3):119–122
Glezeva N, Collier P, Voon V, Ledwidge M, McDonald K, Watson C, Baugh J (2013) Attenuation of monocyte chemotaxis-a novel anti-inflammatory mechanism of action for the cardio-protective hormone b-type natriuretic peptide. Journal of cardiovascular translational research 6(4):545–557
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362(9386):777–781
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27(19):2338–2345
Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, Vardas PE, Bohm M, Dei Cas L (2012) Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail 14(2):219–225
Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP (2007) Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 369(9579):2079–2087
Kindermann M, Reil JC, Pieske B, van Veldhuisen DJ, Bohm M (2008) Heart failure with normal left ventricular ejection fraction: what is the evidence? Trends Cardiovasc Med 18(8):280–292
Ridker PM (2003) Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation 108(19):2292–2297
Gong KZ, Song G, Spiers JP, Kelso EJ, Zhang ZG (2007) Activation of immune and inflammatory systems in chronic heart failure: novel therapeutic approaches. Int J Clin Pract 61(4):611–621
Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM (2009) Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 11(2):119–129
Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A (2003) Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodelling and failure after experimental myocardial infarction. Circulation 108(17):2134–2140
Westermann D, Savvatis K, Lindner D, Zietsch C, Becher PM, Hammer E, Heimesaat MM, Bereswill S, Volker U, Escher F, Riad A, Plendl J, Klingel K, Poller W, Schultheiss HP, Tschope C (2011) Reduced degradation of the chemokine MCP-3 by matrix metalloproteinase-2 exacerbates myocardial inflammation in experimental viral cardiomyopathy. Circulation 124(19):2082–2093
Nishio R, Matsumori A, Shioi T, Ishida H, Sasayama S (1999) Treatment of experimental viral myocarditis with interleukin-10. Circulation 100(10):1102–1108
Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD (2003) Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci (Lond) 105(1):45–50
Bolger AP, Sharma R, von Haehling S, Doehner W, Oliver B, Rauchhaus M, Coats AJ, Adcock IM, Anker SD (2002) Effect of interleukin-10 on the production of tumor necrosis factor-alpha by peripheral blood mononuclear cells from patients with chronic heart failure. Am J Cardiol 90(4):384–389
Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH (2001) Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation 104(12 Suppl 1):I303–I308
Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW (2010) Interleukin-1 blockade with Anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 105(10):1371–1377 e1371
Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR (2004) Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110(22):3480–3487
Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L (2005) Loss or inhibition of uPA or MMP-9 attenuates LV remodelling and dysfunction after acute pressure overload in mice. Am J Pathol 166(1):15–25
Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K (2009) Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail 11(2):191–197
Spinale FG (2007) Myocardial matrix remodelling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87(4):1285–1342
Cox MJ, Hawkins UA, Hoit BD, Tyagi SC (2004) Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation 109(17):2123–2128
Li YY, Kadokami T, Wang P, McTiernan CF, Feldman AM (2002) MMP inhibition modulates TNF-alpha transgenic mouse phenotype early in the development of heart failure. Am J Physiol Heart Circ Physiol 282(3):H983–H989
Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119(16):2209–2216
Gu Y, Lee HM, Sorsa T, Simon SR, Golub LM (2010) Doxycycline [corrected] inhibits mononuclear cell-mediated connective tissue breakdown. FEMS Immunol Med Microbiol 58(2):218–225
Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, Iwanari H, Sagara M, Tanaka T, Hamakubo T, Kodama T, Naito M (2008) Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 215(1):48–55
Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A (2004) Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 110(16):2349–2354
Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A (2008) Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 117(8):1055–1064
Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E (2004) PI3 K gamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118(3):375–387
Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D (2006) Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci USA 103(52):19866–19871
Bujak M, Frangogiannis NG (2009) The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp 57(3):165–176
Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM (2007) Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA 104(43):16810–16815
Liu LC, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK, Sillje HH, van Gilst WH, Jaarsma T, de Boer RA (2011) Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 13(6):619–625
Westermann D, Becher PM, Lindner D, Savvatis K, Xia Y, Frohlich M, Hoffmann S, Schultheiss HP, Tschope C (2012) Selective PDE5A inhibition with sildenafil rescues left ventricular dysfunction, inflammatory immune response and cardiac remodeling in angiotensin II-induced heart failure in vivo. Basic Res Cardiol 107(6):308
Guazzi M, Vicenzi M, Arena R, Guazzi MD (2011) Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124(2):164–174
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E (2013) Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309(12):1268–1277
Conflict of interest
The authors Dr. Nadezhda Glezeva and Dr. John A. Baugh have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glezeva, N., Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev 19, 681–694 (2014). https://doi.org/10.1007/s10741-013-9405-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-013-9405-8