Abstract
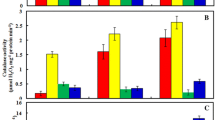
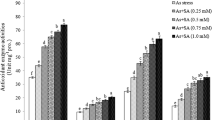
Arsenic (As) toxicity and its biochemical effects have been mostly evaluated in ferns and a few higher plants. In this study, we investigated the effect of As (10.0 and 50.0 μM) on seedling growth, root anatomy, lipid peroxidation (malondialdehyde and conjugated dienes), electrolyte leakage, H2O2 content, root oxidizability and the activities of antioxidant enzymes in mung bean (Phaseolus aureus Roxb.). Arsenic significantly enhanced lipid peroxidation (by 52% at 50.0 μM As), electrolyte leakage and oxidizability in roots. However, there was no significant change in H2O2 content. Arsenic toxicity was associated with an increase in the activities of superoxide dismutase (SOD), guaiacol peroxidase (GPX) and glutathione reductase (GR). In response to 50.0 μM As, the activities of SOD and GR increased by over 60% and 90%, respectively. At 10.0 μM As, the activity of ascorbate peroxidase (APX) increased by 83%, whereas at 50.0 μM it declined significantly. The catalase (CAT) activity, on the other hand, decreased in response to As exposure, and it corresponded to the observed decrease in H2O2 content. We conclude that As causes a reduction in root elongation by inducing an oxidative stress that is related to enhanced lipid peroxidation, but not to H2O2 accumulation.

Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- As:
-
Arsenic
- CAT:
-
Catalase
- CD:
-
Conjugated diene
- DW:
-
Dry weight
- EL:
-
Electrolyte leakage
- FW:
-
Fresh weight
- GPX:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione reduced
- GSSG:
-
Glutathione oxidized
- H2O2 :
-
Hydrogen peroxide
- LP:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reduced
- NBT:
-
Nitro blue tetrazolium
- RO:
-
Root oxidizability
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
- TTC:
-
2,3,5-Triphenyl tetrazolium chloride
References
Armstrong W (1967) The oxidizing activity of roots in waterlogged soils. Physiol Plant 20:920–926
Bajji M, Kinet J-M, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70
Batish DR, Lavanya K, Singh HP, Kohli RK (2007) Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul 51:119–128
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–286
Boveris A, Cadenas E, Chance B (1980). Low level chemiluminescence of the lipoxygenase reaction. Photobiochem Photobiophys 1:175–182
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under lead and arsenic phytotoxicity. Curr Sci 87:342–348
Doyle MO, Otte ML (1997) Organism-induced accumulation of iron, zinc and arsenic in wetland soils. Environ Pollut 96:1–11
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta 157:224–232
Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gajewska E, Slaba M, Andrzejewska R, Sklodowska M (2006) Nickel-induced inhibition of wheat root growth is related to H2O2 production, but not to lipid peroxidation. Plant Growth Regul 49:95–103
Gonzaga MIS, Santos JAG, Ma LQ (2006) Arsenic phytoextrcation and hyperaccumulation by fern species. Sci Agric 63:90–101
Gratäo PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Halliwell B, Gutteridge JM (1989) The chemistry of oxygen radicals and other derived species. In: Halliwell B, Gutteridge JM (eds) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 22–85
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:13–22
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kapustka LA, Lipton J, Galbraith H, Cacela D, Lejeune K (1995) Metallic and arsenic impacts to soils, vegetation communities and wildlife habitat in southwest Montana uplands contained by smelter emissions: II. Laboratory phytotoxicity studies. Environ Toxicol Chem 14:1905–1912
Kedrova L, Saveljev J, Sheshegova T, Shirokhih I, Lisitsyn E (2003) Selection of winter rye (Secale cereale L.) for aluminum and acid resistance. Plant Breed Seed Sci 48:163–168
Li W-X, Chen T-B, Huang Z-C, Lei M, Liao X-Y (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809
Lowry OH, Rosebrough NT, Farr AL, Randall RJ (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Ma B, Wan J, Shen Z (2007) H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regul (in press). DOI 10.1007/s10725-007-9183-1
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelly ED (2001) A fern that hyperaccumulate arsenic. Nature 409:579
Mahimairaja S, Bolan NS, Adriano DC, Robinson B (2005) Arsenic contamination and its risk management in complex environmental settings. Adv Agron 86:1–82
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Matshullat J (2000) Arsenic in the geosphere—a review. Sci Total Environ 249:297–312
Meharg AA (2003) Variation in arsenic accumulation—hyperaccumulation in ferns and their allies. New Phytol 157:25–31
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic-resistant and nonresistant plant species. New Phytol 154:29–43
Montillet J-L, Chamnongpol S, Rustérucci C, Dat J, Van de Cotte B, Agnel J-P, Battesti C, Inzé D, Van Breusegem F, Triantaphylidès C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Päivöke AEA, Simola LK (2001) Arsenate toxicity to Pisum sativum: Mineral nutrients, chlorophyll content, and phytase activity. Ecotoxicol Environ Safety (Environ Res Section B) 49:111–121
Pearce F (2003) Arsenic’s fatal legacy grows. New Sci 179:4–5
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Shaibur MR, Kitajima N, Sugawara R, Kondo T, Huq SMI, Kawai S (2006) Physiological and mineralogical properties of arsenic-induced chlorosis in rice seedlings grown hydroponically. Soil Sci Plant Nutr 52:691–700
Sheppard SC (1992) Summary of phytotoxic levels of soil arsenic. Water Air Soil Pollut 64:539–550
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyperaccumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxidant Redox Signal 8:243–270
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, Inzé D, Breusegem FV (2003) A comprehensive analysis of H2O2-induced gene expression in tobacco. Proc Natl Acad Sci USA 100:16113–16118
Van den Broeck K, Vendecasteele C, Geuns JMC (1998) Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for the arsenic contamination. Anal Chim Acta 361:101–111
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Vitória AP, Da Cunha M, Azevedo RA (2006) Ultrastructural changes of radish leaf exposed to cadmium. Environ Exp Bot 58:47–52
Acknowledgement
Komal Arora is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance in the form of a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.P., Batish, D.R., Kohli, R.K. et al. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53, 65–73 (2007). https://doi.org/10.1007/s10725-007-9205-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-007-9205-z