Abstract
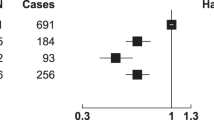
The lifetime prevalence of kidney stones is around 10 % and incidence rates are increasing. Diet may be an important determinant of kidney stone development. Our objective was to investigate the association between diet and kidney stone risk in a population with a wide range of diets. This association was examined among 51,336 participants in the Oxford arm of the European Prospective Investigation into Cancer and Nutrition using data from Hospital Episode Statistics in England and Scottish Morbidity Records. In the cohort, 303 participants attended hospital with a new kidney stone episode. Cox proportional hazards regression was performed to calculate hazard ratios (HR) and their 95 % confidence intervals (95 % CI). Compared to those with high intake of meat (>100 g/day), the HR estimates for moderate meat-eaters (50–99 g/day), low meat-eaters (<50 g/day), fish-eaters and vegetarians were 0.80 (95 % CI 0.57–1.11), 0.52 (95 % CI 0.35–0.8), 0.73 (95 % CI 0.48–1.11) and 0.69 (95 % CI 0.48–0.98), respectively. High intakes of fresh fruit, fibre from wholegrain cereals and magnesium were also associated with a lower risk of kidney stone formation. A high intake of zinc was associated with a higher risk. In conclusion, vegetarians have a lower risk of developing kidney stones compared with those who eat a high meat diet. This information may be important to advise the public about prevention of kidney stone formation.
Similar content being viewed by others
References
Boyce C, Pickhardt P, Lawrence E, Kim D, Bruce R. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol. 2010;183(3):1017–21.
Stamatelou K, Francis M, Jones C, Nyberg L, Curhan G. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–23.
Akoudad S, Szklo M, McAdams M, Fulop T, Anderson C, Coresh J, et al. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: The ARIC study. Prev Med. 2010;51(5):416–20.
Romero V, Akpinar H, Assimos D. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86–96.
Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int. 2012;109(7):1082–7.
Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996;155(3):839–43.
Taylor E, Stampfer M, Curhan G. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455–62.
Taylor E, Fung T, Curhan G. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20(10):2253–9.
Asplin J. Obesity and urolithiasis. Adv Chronic Kidney Dis. 2009;16(1):11–20.
Saigal C, Joyce G, Timilsina A, Project UDiA. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68(4):1808–14.
Worcester E, Coe F. Clinical practice. Calcium kidney stones. N Engl J Med. 2010;363(10):954–63.
Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6(3):259–69.
Bingham SA, Gill C, Welch A, Day K, Cassidy A, Khaw KT, et al. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72(4):619–43.
Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–5.
Nguyen Q, Kälin A, Drouve U, Casez J, Jaeger P. Sensitivity to meat protein intake and hyperoxaluria in idiopathic calcium stone formers. Kidney Int. 2001;59(6):2273–81.
Kok D, Iestra J, Doorenbos C, Papapoulos S. The effects of dietary excesses in animal protein and in sodium on the composition and the crystallization kinetics of calcium oxalate monohydrate in urines of healthy men. J Clin Endocrinol Metab. 1990;71(4):861–7.
Breslau N, Brinkley L, Hill K, Pak C. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66(1):140–6.
Curhan G, Willett W, Rimm E, Stampfer M. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328(12):833–8.
Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497–504.
Curhan G, Willett W, Knight E, Stampfer M. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004;164(8):885–91.
Hess B, Michel R, Takkinen R, Ackermann D, Jaeger P. Risk factors for low urinary citrate in calcium nephrolithiasis: low vegetable fibre intake and low urine volume to be added to the list. Nephrol Dial Transpl. 1994;9(6):642–9.
Jahnen A, Heynck H, Gertz B, Classen A, Hesse A. Dietary fibre: the effectiveness of a high bran intake in reducing renal calcium excretion. Urol Res. 1992;20(1):3–6.
Meschi T, Maggiore U, Fiaccadori E, Schianchi T, Bosi S, Adorni G, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66(6):2402–10.
Siener R, Schade N, Nicolay C, von Unruh G, Hesse A. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol. 2005;173(5):1601–5.
Li MK, Blacklock NJ, Garside J. Effects of magnesium on calcium oxalate crystallization. J Urol. 1985;133(1):123–5.
Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol. 2000;163(5):1565–9.
Tang J, McFann K, Chonchol M. Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol. 2012;36(6):549–53.
Trinchieri A, Mandressi A, Luongo P, Rovera F, Longo G. Urinary excretion of citrate, glycosaminoglycans, magnesium and zinc in relation to age and sex in normal subjects and in patients who form calcium stones. Scand J Urol Nephrol. 1992;26(4):379–86.
Carpentier X, Bazin D, Combes C, Mazouyes A, Rouzière S, Albouy PA, et al. High Zn content of Randall’s plaque: a μ-X-ray fluorescence investigation. J Trace Elem Med Biol. 2011;25(3):160–5.
Acknowledgments
EPIC-Oxford is supported by Cancer Research UK (formerly the Imperial Cancer Research Fund), the Medical Research Council and the European Commission. BT was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford. None of these funding sources contributed directly to this study.
Conflict of interest
None.
Ethical standard
EPIC-Oxford is registered under the Data Protection Act, has ethical approval from Scotland A Research Ethics Committee (MREC/02/0/90), and has approval for the collection of clinical and pathological NHS data from National Information Governance Board under Sect. 251 (PIAG3-09(e)/2003 and PIAG 1-05(d)/2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turney, B.W., Appleby, P.N., Reynard, J.M. et al. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol 29, 363–369 (2014). https://doi.org/10.1007/s10654-014-9904-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9904-5