Abstract
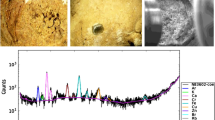
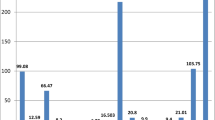
In view of the high incidence rate of urinary stones in the south and southwest of Iran, this paper investigates trace elements content including heavy metals in 39 urinary stones, collected from patients in Fars province, Iran. The mineralogy of the stones is investigated using X-ray diffractometry. The samples are classified into five mineral groups (calcium oxalate, uric acid, cystine, calcium phosphate and mixed stone). Major and trace elements in each group were determined using ICP-MS method. P and Ca constitute the main elements in urinary stones with Ca being more affine to oxalates while other alkali and alkaline earths precipitate with phosphate. Significant amounts of trace elements, especially Zn and Sr, were found in urinary calculi (calcium oxalate and phosphates) relative to biominerals (uric acid and cystine). Among urinary calculi, calcium phosphate contains greater amounts of trace metal than calcium oxalate. Phosphates seem to be the most important metal-bearing phases in urinary stones. Results indicate that concentrations of elements in urinary stones depend on the type of mineral phases. Significant differences in elements content across various mineralogical groups were found by applying statistical methods. Kruskal–Wallis test reveals significant difference between Ca, P, K, Na, Mg, S, Zn, Sr, Se, Cd, and Co content in different investigated mineral groups. Moreover, Mann–Whitney test differentiates Ca, Na, Zn, Sr, Co, and Ni between minerals in oxalate and uric acid stones. This study shows that urinary stone can provide complementary information on human exposure to elements and estimate the environmental risks involved in urinary stones formation.




Similar content being viewed by others
References
Abboud, I. A. (2008a). Analyzing correlation coefficients of the concentrations of trace elements in urinary stones. Jordan Journal of Earth and Environmental Sciences, 1(2), 73–80.
Abboud, I. A. (2008b). Concentration effect of trace metals in Jordanian patients of urinary calculi. Environmental Geochemistry and Health, 30(1), 11–20.
Abboud, I. A. (2008c). Mineralogy and chemistry of urinary stones: patients from North Jordan. Environmental Geochemistry and Health, 30(5), 445–463.
Abed, A. M., & Abdalla, R. S. (1998). On the state of weathering of the Upper Cretaceous red phosphorites of Eshidiya, southern Jordan. Journal of African Earth Sciences, 27(1), 39–54.
Ackermann, D., Baumann, J. M., Futterlieb, A., & Zingg, E. J. (1988). Influence of calcium content in mineral water on chemistry and crystallization conditions in urine of calcium stone formers. European Urology, 14(4), 305–308.
Andrew, J. P. & Chandru, P. S. (2001). Diagnosis and initial management of kidney stones. American Family Physician, http://www.aatp.org/atp/20010401/1329.html. p. 17.
Atakan, I. H., Kaplan, M., Seren, G., Aktoz, T., Gül, H., & Inci, O. (2007). Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. International Urology and Nephrology, 39(2), 351–356.
Basiri, A., Shakhssalim, N., Khoshdel, A. R., Ghahestani, S. M., & Basiri, H. (2010). The demographic profile of urolithiasis in Iran: a nationwide epidemiologic study. International Urology and Nephrology, 42(1), 119–126.
Bazin, D., Carpentier, X., Traxer, O., Thiaudiere, D., Somogyi, A., Reguer, S., et al. (2008). Very first tests on SOLEIL regarding the Zn environment in pathological calcifications made of apatite determined by X-ray absorption spectroscopy. Journal of Synchrotron Radiation, 15(5), 506–509.
Bazin, D., Chevallier, P., Matzen, G., Jungers, P., & Daudon, M. (2007). Heavy elements in urinary stones. Urological Research, 35(4), 179–184.
Bazin, D., Daudon, M., Combes, C., & Rey, C. (2012). Characterization and some physicochemical aspects of pathological microcalcifications. Chemical Reviews, 112(10), 5092–5120.
Beging, S., Mlynek, D., Hataihimakul, S., Poghossian, A., Baldsiefen, G., Busch, H., et al. (2010). Field-effect calcium sensor for the determination of the risk of urinary stone formation. Sensors and Actuators B: Chemical, 144(2), 374–379.
Bellizzi, V., DeNicola, L., Minutolo, R., Russo, D., Cianciaruso, B., Andreucci, M., et al. (1999). Effects of water hardness on urinary risk factors for kidney stones in patients with idiopathic nephrolithiasis. Nephron, 81(Suppl. 1), 66–70.
Brikowski, T. H., Lotan, Y., & Pearle, M. S. (2008). Climate-related increase in the prevalence of urolithiasis in the United States. Proceedings of the National Academy of Sciences, 105(28), 9841–9846.
Chandrajith, R., Wijewardana, G., Dissanayake, C., & Abeygunasekara, A. (2006). Biomineralogy of human urinary calculi (kidney stones) from some geographic regions of Sri Lanka. Environmental Geochemistry and Health, 28(4), 393–399.
Dajani, A., Khadra, A. A., & Baghdadi, F. (1988). Urolithiasis in Jordanian Children.: A Report of 52 Cases. British Journal of Urology, 61(6), 482–486.
Deeming, S., & Weber, C. (1977). Evaluation of hair analysis for determination of zinc status using rats. The American Journal of Clinical Nutrition, 30(12), 2047–2052.
Esteban, M., & Castaño, A. (2009). Non-invasive matrices in human biomonitoring: a review. Environment International, 35(2), 438–449.
Evan, A. P. (2010). Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatric Nephrology, 25(5), 831–841.
Failla, M. L. (2003). Trace elements and host defense: recent advances and continuing challenges. The Journal of Nutrition, 133(5), 1443S–1447S.
Fang, X., Ahmad, S. R., Mayo, M., & Iqbal, S. (2005). Elemental analysis of urinary calculi by laser induced plasma spectroscopy. Lasers in Medical Science, 20(3–4), 132–137.
Fraga, C. G. (2005). Relevance, essentiality and toxicity of trace elements in human health. Molecular Aspects of Medicine, 26(4), 235–244.
Giannossi, M. L., & Summa, V. (2013). An observation on the composition of urinary calculi: environmental influence. Medical geochemistry (pp. 67–90). Netherlands: Springer.
Giannossi, M. L., Summa, V., & Mongelli, G. (2013). Trace element investigations in urinary stones: A preliminary pilot case in Basilicata (Southern Italy). Journal of Trace Elements in Medicine and Biology, 27(2), 91–97.
Golovanova, O., Palchik, N., Maksimova, N., & In, A. (2006). Comparative characterization of the microelement composition of kidney stones from patients in the Novosibirsk and Omsk regions.
Hesse, A., & Sanders, G. (1988). Atlas of infrared spectra for the analysis of urinary concrements. Stuttgart: Georg Thieme.
Hesse, A., Tiselius, H. G., Siener, R., & Hoppe, B. (2009). Urinary stones: diagnosis, treatment, and prevention of recurrence. 3rd revised and enlarged edition. Karger: Basel.
Hofbauer, J., Steffan, I., Höbarth, K., Vujicic, G., Schwetz, H., Reich, G., et al. (1991). Trace elements and urinary stone formation: new aspects of the pathological mechanism of urinary stone formation. The Journal of Urology, 145(1), 93–96.
Houtman, J. P. (1996). Trace elements and cardiovascular diseases. Journal of Cardiovascular Risk, 3(1), 18–24.
Jing, Z., GuoZeng, W., Ning, J., JiaWei, Y., Yan, G., & Fang, Y. (2010). Analysis of urinary calculi composition by infrared spectroscopy: a prospective study of 625 patients in eastern China. Urological Research, 38(2), 111–115.
Joost, J., & Tessadri, R. (1986). Trace element investigations in kidney stone patients. European Urology, 13(4), 264–270.
Kohri, K., Garside, J., & Blacklock, N. (1988). The role of magnesium in calcium oxalate urolithiasis. British Journal of Urology, 61(2), 107–115.
Kohri, K., Kodama, M., Ishikawa, Y., Katayama, Y., Takada, M., Katoh, Y., et al. (1989). Magnesium-to-calcium ratio in tap water, and its relationship to geological features and the incidence of calcium-containing urinary stones. The Journal of Urology, 142(5), 1272–1275.
Kuta, J., Machát, J., Benová, D., Červenka, R., & Kořistková, T. (2012). Urinary calculi—atypical source of information on mercury in human biomonitoring. Central European Journal of Chemistry, 10(5), 1475–1483.
Kuta, J., Machát, J., Benová, D., Červenka, R., Zeman, J., & Martinec, P. (2013). Association of minor and trace elements with mineralogical constituents of urinary stones: A hard nut to crack in existing studies of urolithiasis. Environmental Geochemistry and Health, 35, 1–12.
Lentner, C. (1981). Geigy scientific tables. English edition. Vol. 1 (Vol. 53–107). Ciba Geigy: Basel.
Levinson, A., Nosal, M., Davidman, M., Prien, E, Sr, Prien, E, Jr, & Stevenson, R. (1978). Trace elements in kidney stones from three areas in the United States. Investigative Urology, 15(4), 270.
Mertz, W. (1981). The essential trace elements. Science, 213(4514), 1332–1338.
Meyer, J. L., & Thomas, W. C, Jr. (1982). Trace metal-citric acid complexes as inhibitors of calcification and crystal growth. I. Effects of Fe(III), Cr(III) and Al(III) complexes on calcium phosphate crystal growth. The Journal of Urology, 128(6), 1372–1375.
Munoz, J. A., & Valiente, M. (2005). Effects of trace metals on the inhibition of calcium oxalate crystallization. Urological Research, 33(4), 267–272.
Nasir, S., Kassem, M. E., El-Sherif, A., Fattah, T. (2004). Physical investigation of urinary calculi: Example from the Arabian Gulf (State of Qatar). http://www.oxfordresearchforum.i12.com/articles/article% 205.htm (assessed on 12.08.2005).
Navarro Silvera, S. A., & Rohan, T. E., (2007). Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control, 18, 7–27.
Perk, H., Serel, T. A., Koşar, A., Deniz, N., & Sayin, A. (2002). Analysis of the trace element contents of inner nucleus and outer crust parts of urinary calculi. Urologia Internationalis, 68(4), 286–290.
Pourmand, G., & Pourmand, B. (2012). Epidemiology of stone disease in Iran. In urolithiasis (Vol. 85–87). London: Springer.
Robertson, W. G. (2010). Urinary tract stones in: medical therapy in urology. Springer: London.
Robertson, W. G., Peacock, M., Heyburn, P., & Hanes, F. (1980). Epidemiological risk factors in calcium stone disease. Scandinavian Journal of Urology and Nephrology, 53, 15–28.
Pearle. M. S, Yair Lotan, (2012), Urinary Lithiasis: Etiology, epidemiology, and pathogenesis in: Campbell-Walsh urology, 10thed.
Safarinejad, M. R. (2007). Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urological Research, 35(2), 73–82.
Sakly, R., Chaouch, A., El Hani, A., & Najjar, M.-F. (2003). Effects of intraperitoneally administered vitamin E and selenium on calcium oxalate renal stone formation: experimental study in rat. Paper presented at the Annales d’urologie.
Schubert, G. (2006). Stone analysis. Urological Research, 34(2), 146–150.
Shannon, R. T., & Prewitt, C. T. (1969). Effective ionic radii in oxides and fluorides. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 25(5), 925–946.
Shokouhi, B., Gasemi, K., & Norizadeh, E. (2008). Chemical composition and epidemiological risk factors of urolithiasis in Ardabil Iran. Research Journal of Biological Sciences, 3(6), 620–626.
Singh, V. K., Rai, A., Rai, P., & Jindal, P. (2009). Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Lasers in Medical Science, 24(5), 749–759.
Słojewski, M. (2011). Major and trace elements in lithogenesis. Central European Journal of Urology, 64(2), 58–61.
Słojewski, M., Czerny, B., Safranow, K., Jakubowska, K., Olszewska, M., Pawlik, A., et al. (2010). Microelements in stones, urine, and hair of stone formers: a new key to the puzzle of lithogenesis? Biological Trace Element Research, 137(3), 301–316.
Sobhi, N. (2006). “The mineralogy and chemistry of urinary stones from the Arabian Gulf.” Internet site, work not published., Result work under proposing: 8.
Stamatelou, K. K., Francis, M. E., Jones, C. A., Nyberg, L. M., & Curhan, G. C. (2003). Time trends in reported prevalence of kidney stones in the United States: 1976–19941. Kidney International, 63(5), 1817–1823.
Stranges, S., Marshall, J. R., Natarajan, R., Donahue, R. P., Trevisan, M., Combs, G. F., et al. (2007). Effects of long-term selenium supplementation on the incidence of type 2 diabetes a randomized trial. Annals of Internal Medicine, 147(4), 217–223.
Sutor, D. (1969). Growth studies of calcium oxalate in the presence of various ions and compounds. British Journal of Urology, 41(2), 171–178.
Touryan, L. A., Lochhead, M. J., Marquardt, B. J., & Vogel, V. (2004). Sequential switch of biomineral crystal morphology using trivalent ions. Nature Materials, 3(4), 239–243.
Tur, J., Prieto, R., & Grases, F. (1991). An animal model to study the effects of diet on risk factors of calcium stone formation. Scandinavian Journal of Urology and Nephrology, 25(4), 311–314.
Wandt, M. A. E., & Underhill, L. G. (1988). Covariance biplot analysis of trace element concentrations in urinary stones. British Journal of Urology, 61(6), 474–481.
Zarasvandi, A., Heidari, M., Sadeghi, M., & Mousapoor, E. (2013). Major and trace element composition of urinary stones, Khuzestan province, Southwest, Iran. Journal of Geochemical Exploration, 131, 52–58.
Acknowledgments
This research was supported by “Shiraz University Medical Geology Research” to whom the authors are indebted. The authors would also like to express their gratitude to Zarazma Mineral Studies Company for their sample analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshavarzi, B., Yavarashayeri, N., Irani, D. et al. Trace elements in urinary stones: a preliminary investigation in Fars province, Iran. Environ Geochem Health 37, 377–389 (2015). https://doi.org/10.1007/s10653-014-9654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9654-z