Abstract
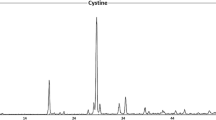
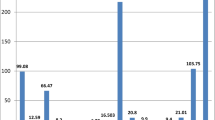
Due to the increase in the number of urinary calculi disease cases in Jordan, stone samples were collected from patients from various Jordanian hospitals (Princes Basma (PBH), King Abdullah University (KAUH), Al-Basheer (ABH) and Al-Mafraq (AMH)). This study concentrates on the effect of trace metals in patients of urinary calculi. Trace metals were detected in 110 urinary calculi samples using X-ray fluorescence (XRF) and atomic absorption spectroscopy (AAS) techniques. Of the calculi examined, 21 were pure calcium oxalate (CaOax), 29 were mixed calcium oxalate/uric acid, 23 were mixed calcium oxalate/phosphate (apatite), 25 were phosphate calculi (apatite/struvite), five were mixed calcium oxalate monohydrate/struvite, four were urate calculi (mixed ammonium acid urate/sodium acid urate) and three were pure cystine calculi. The concentration measurement of Ca and other trace metals levels has been found useful in understanding the mechanism of stone formation and in evaluating pathological factors. It has been found that Ca is the main constituent of the urinary calculi, especially those stones composed of calcium oxalate and calcium phosphate. The concentration of most of the trace metals that were analyzed was (Ca = 48.18, Na = 1.56, K = 0.9, Mg = 3.08, Fe = 1.17, Al = 0.49, Zn = 0.7, Cu = 0.19, Mn = 0.029, P = 10.35, S = 1.88, Sr = 0.306, Mo = 0.2, Cr = 0.146, Co = 0.05, Ni = 0.014)%. In conclusion, metals concentration in Jordanian patient’s urinary calculi samples was higher than its equivalents of other patients’. It has been noted that there is no concentration of toxic trace elements (like Li, V, Pb, Cd, and As). Some heavy metals, however, were detected Mo, Cr, Co and Ni as traces. P and S ions are present in few calculi stones as traces.
Similar content being viewed by others
References
Abboud, I. A. (2006). Mineralogy & Chemistry of Urinary Stones – Patients from North Jordan – Study in Medical Geochemistry. Al al-Bayt University Projects. Result work under proposing.
Abdel-Halim, R. E., Al-Sibaai, A., & Baghlff, A. O. (1993). Ionic associations within 460 non infection urinary stones. Scandinavian Journal of Urology and Nephrology, 27, 155–162.
Abu-Farsakh, F. (1997). Correlation between copper, zinc and some lipids in serum, bile and stones of patients with gall stone disease. Dirasat, Medical and Biological Sciences, 24(1), 54–59.
Al-Kinani, A. T., Watt, D. E., East, B. W. et al. (1984). Minor and trace element analysis of gallstones. Analyst, 109, 365–368.
Al-Fawaaz, M. M. (2006). Diagnostic of Environmental Effects in Stone Formation and Traces on Human Health – Northeastern of Jordan, Study in Medical Geochemistry. M. Sc. Thesis, Al al-Bayt University, Jordan, P124.
Al-Maliki, M. A. (1998). Renal Stones a Study in medical geochemistry. M. Sc. Thesis, Baghdad University, p. 101.
Batanjac, J. (2000). Nutritional aspects in oxalic urolithiasis. The Scientific Journal FACTA UNIVERSITATIS, Series: Medicine and Biology, 7(1), 49–51.
Chutipongtanate, C., Nakagawa, Y., Sritippayawan, S., et al. (2005). Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. The Journal of Clinical Investigation, 115, 3613–3622.
Dajani, A., Abu Khadra, A., Baghdadi, F. (1988). Urolithiasis in Jordanian children. A report of 52 cases. British Journal of Urology, 61, 482–486.
Deeming, S. P., & Wepu, C. W. (1977). Evaluation of hair analysis for determination of zinc status using rats. The American Journal of Clinical Nutrition, 30, 2047–2052.
Durak, I., Yasar, A., Yurtarslani, Z., Akpoyraz, M., & Tasman, S. (1988). Analysis of magnesium and trace elements in urinary calculi by Atomic absorption spectrophotometry. British Journal of Urology, 62, 203–205.
Evenson, M. A., & Warren, B. L. (1975). Determination of copper by atomic absorption, with use of the graphic Cuvette. Clinical Chemistry, 21, 619–625.
Feinendegen, L. E. & Kasperek, K. (1980). Medical aspects of trace element research. In Bratter, P. & Schramel, P. (Eds.), Trace Element Analytical Chemistry in Medicine and Biology. Proceedings of first international workshop (pp. 1–37). Berlin: de Gruyter.
Fell, G. S. (1984). Accuracy of trace element analysis in biological samples. Tr AC, Trends in Analytical Chemistry, 4, IX–X.
Foote, J. W., & Delves, H. T. (1982). Determination of zinc in small volumes of serum using absorption spectrophotometry with electrothermal automization. Analyst, 107, 1729–1734.
Fullerton, H. (2003). Transposition of directive 2002/46/EC in food supplements: draft food supplements regulations (WALES). PhD Thesis, 5 Bryngelli, Carmel, Llanelli, Carms. SA14 7TL. P 18. http://www.alliance-natural-health.org/_docs/ANHwebsiteDoc_4-.com.
Jhaumeer-Laulloo, S. & Subratty, A.H. (1999). Analysis of urinary calculi in Mauritius. Science & Technology- Research Journal, 3, 87–93.
Joost, J., Tessadri, R. (1987). Trace elements investigations in kidney stone patients. Europe Urology, 13, 264–270.
Hammarsten, G. (1929). On calcium oxalate and its solubility in the presence of inorganic salts with special references to the occurrence of oxaluria. Compt. Rend. Trav. Lab. Carlsberg, 17, 1–85. In Wandt, M. A. E. & Underhill, L. G. (1988). Covariance biplot analysis of trace element concentrations in urinary stones. British Journal of Urology, 61, 474–481.
Harte, J. et al. (1991). Toxics A to Z: A guide to everyday pollution hazards. Berkeley, CA: University of California Press, p. 103.
Karlsen, J. S., Grenabo, L., & Holmberg, G. (1995). A new system for descriptive classification stones in the upper urinary tract. Journal of Urology, 153, 378–379.
Kellas, B. & Dworkin, A. (1996). Surviving the toxic crisis. Olivenhain, CA: Professional Preference Publishing, 187. 217, 230–234.
King, J., Jr. (1971). Currents in renal stones research. Clinical Chemistry, 17, 971–982.
Levinson, A. A., Nosa, M., Davidman, A. et al. (1978). Trace elements in kidney stones from three areas in the United States. Investigative Urology, 15, 270–274.
Lieske, J., Leonard, R., & Toback, G. (1995). Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. American Journal of Physiology, Renal, Fluid and Electrolyte Physiology, 37(4), 604–612.
Malet, P. F., Williamson, C. E., Trotman, B. W., & Soloway, R. D. (1986). Composition of pigmented centers of cholesterol gall stones. Hepatology, 6, 477–481.
Mena, J. et al. (1969). Chronic manganese poisoning. Neurology, 19, 1000–1006.
Meranger, J.C. & Smith, D.C. (1972). The heavy metals content of a typical Canadian diet. Canadian Journal of Public Health, 63 pp. 53.
Meyer, J. L., & Angino, E. F. (1977). The role of trace metals in calcium urolithiasis. Investigative Urology, 14, 347–350.
Meyer, J. L., & Thomas, W. C., Jr. (1982). Trace metal-citric acid complexes as inhibitors of calcification and crystal growth. II. Effects of Fe(III), Cr(III) and Al(III) complexes on calcium oxalate crystal growth. Journal of Urology, 128, 1376–1378.
Mhelan, M. M. (1992). The management and treatment of 400 patients with urolithiasis. Dirasat Journal, V19 (B), No. 4.
Neithereut, W. D. (1989). Effect of calcium, magnesium and sodium ions on vitro nucleation of human gall bladder bile. Gut, 30, 665–670.
Neuman, W. F., & Neuman, M. W. (1953). The nature of the mineral phase of bone. Chemical Reviews, 53, 1–45.
Ohta, N. (1957). Studies on inorganic constituents in biological materials-On the inorganic constituents in human stones. Bulletin of the Chemical Society of Japan, 30, 833–841.
Pouls, M., & Payne, M. (2006). Oral chelation and nutritional replacement therapy for heavy metal toxicity and cardiovascular conditions. Manuscript (written by Extreme Health), Publishing by the University of Michigan. www.extremehealthusa.com.
Reynolds, T. M. (2005). Chemical pathology clinical investigation and management of nephrolithiasis. Journal of Clinical Pathology, 58, 134–140.
Robertson, W. G., Peacock, M., Heyburn, P., & Hanes, F., (1980). Epidemionological risk factors in calcium stone disease. Scandinavian Journal of Urology and Nephrology, 53, 15–28.
Samuell, C. T., & Kasidas, G. P. (1995). Biochemical investigations in renal stone formers. Annals of Clinical Biochemistry, 32(2), 112–122.
Saw, K. C., McAteer, J. A., Monga, A. G., et al. (2000). Effect of stone composition, stone size, and scan collimation. American Journal of Roentgenology, 175, 329–332.
Schneider, H. -J., Straube, G., & Anke, M. (1970). Zink in harnsteinen. Zeitschrift für Urologie, 63, 895–900.
Schwille, P. O., Hamper, A., & Sigel, A. (1985). Urinary and serum sulfate in idiopathic recurrent calcium urolithiasis. In Urolithiasis and Related Clinical Research. Proceedings of the fifth international symposium (pp. 339–342) New York: Plenum.
Scott, R., Cunningham, C., McLelland, A. et al. (1982). The importance of cadium as a factor in calcified upper urinary tract stone disease-a prospective 7-years study. British Journal of Urology, 54, 584–589.
Simpson, D. R. (1968). Substitution in apatite: I. Potassium-bearing apatite. The American Mineralogist, 53, 432–444.
Sobel, A. E., Nobel, S., Hanok, A. (1949). The reversible inactivation of calcification in virto. Proceedings of the Society for Experimental Biology and Medicine, 72, 68–72. In Wandt, M.A.E. and Underhill, L.G. (1988). Covariance biplot analysis of trace element concentrations in urinary stones. British Journal of Urology, 61, 474–481.
Sobhi, (2006). The mineralogy and chemistry of urinary stones from the Arabian Gulf. Internet site, work not published, Result work under proposing, pp. 8.
Sutor, D. J. (1969). Growth studies of calcium oxalate in the presence of various ions and compounds. British Journal of Urology, 41, 171–178.
Torzewska, A., Staczek, P., & Rozalski, A. (2003). Crystallization of urine mineral components may depend on the chemical nature of proteus endotoxin polysaccharides. Journal of Medical Microbiology, 52, 471–477.
Vergauwe, D. A., Verbeeck, R. M., & Oosterlinnck, W. (1994). Analysis of urinary calculi. Acta Urology of Belgium, 62(2), 5–13.
Wandt, M. A. E., Pougnet, M. A. B., & Rodgers, A. L. (1984). Determination of calcium, magnesium and phosphors in human stones by inductive coupled plasma atomic-emission spectroscopy. Analyst, 109, 1071–1074.
Wandt, M. A. E., & Underhill, L. G. (1988). Covariance biplot analysis of trace element concentrations in urinary stones. British Journal of Urology, 61, 474–481.
Yagisawa, T., Hayashi, T., Yoshida, A., Okuda, H., Kobayashi, H., Ishikawa, N., Goya, N., & Toma, H. (1999). Metabolic characteristics of the elderly with recurrent calcium oxalate stones. BJU International, 83, 924–928.
Acknowledgements
This research has been sponsored by Al al-Bayt University. The researcher would like to express his appreciation for the help of Prof. Dr. Nadher Al Ansari, Dr. Ali Ahmed Bani Nasser, and Mr. Musa Al-Zghoul for their invaluable comments during the different stages of carrying out of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abboud, I.A. Concentration effect of trace metals in Jordanian patients of urinary calculi. Environ Geochem Health 30, 11–20 (2008). https://doi.org/10.1007/s10653-007-9103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-007-9103-3