Abstract
Vitamin D deficiency has been associated with cholestatic liver disease such as primary biliary cirrhosis. Some studies have suggested that cirrhosis can predispose patients to development of osteoporosis because of altered calcium and vitamin D homeostasis. The aim of this study was to determine the prevalence of vitamin D deficiency in patients with chronic liver disease.
Methods
One hundred and eighteen consecutive patients (43 with hepatitis C cirrhosis, 57 with hepatitis C but no cirrhosis, 18 with nonhepatitis C-related cirrhosis) attending the University of Tennessee Hepatology Clinic had their 25-hydroxyvitamin D level measured. Severity of vitamin D deficiency was graded as mild (20–32 ng/ml), moderate (7–19 ng/ml) or severe (<7 ng/ml), normal being >32 ng/ml.
Results
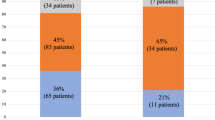
Of patients, 109/118 (92.4%) had some degree of vitamin D deficiency. In the hepatitis C cirrhosis group, 16.3% (7/43) had mild, 48.8% (21/43) had moderate, and 30.2% (13/43) had severe vitamin D deficiency. In the hepatitis C noncirrhotic group, 22.8% (19/57) had mild, 52.6% (30/57) had moderate, and 14% (8/57) had severe vitamin D deficiency. In the nonhepatitis C-related cirrhosis group, 38.9% (7/18) had mild, 27.8% (5/18) had moderate, and 27.8% (5/18) had severe vitamin D deficiency. Severe vitamin D deficiency (<7 ng/ml) was more common among patients with cirrhosis compared with noncirrhotics (29.5% versus 14.1%, P value = 0.05). Female gender, African American race, and cirrhosis were independent predictors of severe vitamin D deficiency in chronic liver disease.
Conclusion
Vitamin D deficiency is universal (92%) among patients with chronic liver disease, and at least one-third of them suffer from severe vitamin D deficiency. African American females are at highest risk of vitamin D deficiency.
Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- CLD:
-
Chronic liver disease
- HCV:
-
Hepatitis C virus
- MMP:
-
Matrix metalloproteinase
- 25(OH)D:
-
25-hydroxyvitamin D
References
DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(Suppl):1689S–1696S.
Imawari M, Akanuma Y, Itakura H, Muto Y, Kosaka K, Goodman DS. The effects of diseases of the liver on serum 25 hydroxyvitamin D and on serum binding protein for vitamin D and its metabolites. J Lab Clin Med. 1979;93:171–180.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281.
Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older US white adults. J Bone Miner Res. 2008;23:143–150.
Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304.
Tsuneoka K, Tameda Y, Takase K, et al. Osteodystrophy in patients with chronic hepatitis and liver cirrhosis. J Gastroenterol. 1996;31:669–678.
Masuda S, Okano T, Osawa K, et al. Concentrations of vitamin D-binding protein and vitamin D metabolites in plasma of patients with liver cirrhosis. J Nutr Sci Vitaminol (Tokyo). 1989;35:225–234.
Bouillon R, Auwerx J, Dekeyser L, et al. Serum vitamin D metabolites and their binding protein in patients with liver cirrhosis. J Clin Endocrinol Metab. 1984;59:86–89.
Chen CC, Wang SS, Jeng FS, et al. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11:417–421.
Hepner GW, Roginsky M, Moo HF. Abnormal vitamin D metabolism in patients with cirrhosis. Am J Dig Dis. 1976;21:527–532.
Duarte MP, Farias ML, Coelho HS, et al. Calcium-parathyroid hormone-vitamin D axis and metabolic bone disease in chronic viral liver disease. J Gastroenterol Hepatol. 2001;16:1022–1027.
Monegal A, Navasa M, Guanabens N, et al. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int. 1997;60:148–154.
Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687.
Koli K, Keski-Oja J. 1alpha, 25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229.
Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796.
Dobak J, Grzybowski J, Liu FT, et al. 1, 25-dihydroxyvitamin D3 increases collagen production in dermal fibroblasts. J Dermatol Sci. 1994;8:18–24.
Garcíade León Mdel C, Montfort I, Tello Montes E, et al. Hepatocyte production of modulators of extracellular liver matrix in normal and cirrhotic rat liver. Exp Mol Pathol. 2006;80(1):97–108. Epub 2005 Dec 5.
Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47(1):186–198.
Khandoga A, Kessler JS, Hanschen M, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006;79:1295–1305.
Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5(4):513–520. Epub 2007 Jan 10.
Mawer EB, Klass HJ, Warnes TW, et al. Metabolism of vitamin D in patients with primary biliary cirrhosis and alcoholic liver disease. Clin Sci (Lond). 1985;69:561–570.
Caniggia A, Lore F, di Cairano G, et al. Main endocrine modulators of vitamin D hydroxylases in human pathophysiology. J Steroid Biochem. 1987;27:815–824.
Skinner RK, Sherlock S, Long RG, et al. 25-hydroxylation of vitamin D in primary biliary cirrhosis. Lancet. 1977;1:720–721.
Compston JE. Hepatic osteodystrophy: vitamin D metabolism in patients with liver disease. Gut. 1986;27:1073–1090.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arteh, J., Narra, S. & Nair, S. Prevalence of Vitamin D Deficiency in Chronic Liver Disease. Dig Dis Sci 55, 2624–2628 (2010). https://doi.org/10.1007/s10620-009-1069-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1069-9