Abstract
Aims
The aim of this study is to assess the efficacy and safety of lubiprostone in adults with chronic constipation.
Methods
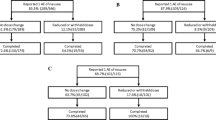
This multicenter, parallel-group trial enrolled 237 patients with chronic constipation and randomized them to 4 weeks of double-blind treatment with oral lubiprostone 24 mcg or placebo twice daily. The primary efficacy endpoint was the number of spontaneous bowel movements (SBMs) after 1 week of treatment. Secondary evaluations included SBMs at weeks 2, 3, and 4; percentage of patients with a SBM within 24 h of first study dose; stool consistency; degree of straining; constipation severity; abdominal bloating and discomfort; global treatment effectiveness; and safety assessments.
Results
Lubiprostone-treated patients experienced greater mean numbers of SBMs at week 1 compared with placebo (5.89 versus 3.99, P = 0.0001), with significantly greater percentages having SBMs within 24 h of the first dose (61.3% versus 31.4%, P < 0.0001). At each assessment, SBM frequency and percentages of full responders (≥4 SBM per week) were significantly greater among lubiprostone-treated patients compared with placebo (P ≤ 0.0171). Lubiprostone-treated patients reported significant improvements in stool consistency, straining, and constipation severity at all weeks, and in abdominal bloating at week 1. Patient assessments of treatment effectiveness were significantly greater with lubiprostone compared with placebo at all weeks (P < 0.0004). Gastrointestinal-related disorders were the most common adverse events in both treatment groups.
Conclusions
In patients with chronic constipation, lubiprostone produced a bowel movement in the majority of individuals within 24 h of initial dosing, with sustained improvement in frequency as well as other constipation symptoms over 4 weeks of treatment.




Similar content being viewed by others
References
Brandt LJ, Prather CM, Quigley EMM, et al. Systematic review on the management of chronic constipation in north america. Am J Gastroenterol. 2005;100:S5–S22.
Stewart W, Liberman J, Sandler RS, et al. Epidemiology of constipation (epoc) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol. 1999;94:3530–3540.
Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759.
Talley NJ, Lasch KL, Baum CL. A gap in our understanding: chronic constipation and its comorbid conditions. Clin Gastroenterol Hepatol. 2009;7(1):9–19.
Johanson JF. Geographic distribution of constipation in the United States. Am J Gastroenterol. 1998;93:188–191.
Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608.
Johanson JF, Gargano MA, Holland PC, et al. Phase III study of lubiprostone, a chloride channel-2 (clc-2) activator for the treatment of constipation: safety and primary efficacy. Am J Gastroenterol. 2005;100(Suppl 9):S328–S329.
Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, four-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator in patients with chronic constipation. Am J Gastroenterol. 2008;103:170–177.
Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther. 2007;25:1351–1361.
Drost J, Harris LA. Diagnosis and management of chronic constipation. JAAPA. 2006;19:24–29.
Camilleri M, Bharaucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G942–G947.
Cuppoletti J, Malinowska DH, Tewari KP, et al. Spi-0211 activates t84 cell chloride transport and recombinant human clc-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–C1183.
Ueno R, Osama H, Habe T, et al. Oral spi-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels (abstract). Gastroenterology. 2004;126:298.
Acknowledgments
Writing and editorial support for this manuscript was provided by Brian G. Shearer, PhD, Takeda Pharmaceuticals North American, Deerfield, IL and Susan Ruffalo, PharmD, MedWrite, Inc., Newport Coast, California.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded, designed, conducted, and supervised by the sponsor, Sucampo Pharma Americas, Inc., Bethesda, MD.
Amitiza® is a registered trademark of Sucampo Pharma Americas, Inc.
Dulcolax® is a registered trademark of Boehringer Ingelheim GmbH.
Fleet Enema® is a registered trademark of C. B. Fleet Company.
SAS® is a registered trademark of SAS Institute Inc.
Rights and permissions
About this article
Cite this article
Barish, C.F., Drossman, D., Johanson, J.F. et al. Efficacy and Safety of Lubiprostone in Patients with Chronic Constipation. Dig Dis Sci 55, 1090–1097 (2010). https://doi.org/10.1007/s10620-009-1068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1068-x