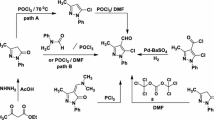
Benzofuro[2,3-b]indolines undergo ring opening in the presence of base to generate 3H-indolines. The latter can rearrange into 3-arylindoles through an intramolecular transfer of the methoxycarbonyl moiety from quaternary carbon to oxygen of phenol. The intermediate 3H-indolines can be isolated upon DMAP-catalyzed O-acylation of the phenol moiety with Boc2O.
















Similar content being viewed by others
Notes
* Isolated compound 11a was converted to N-deprotected indole 8a under thermal conditions (PhMe, 160°C, 30 min)8 to confirm the structural assignment for compound 8a, which was based on the NMR experiments.
* As has been demonstrated by Hassner,9 the reaction of DMAP with Boc2O produces ion pair: N-Boc-pyridinium tert-butoxycarboxylate. The tert-butoxycarboxylate decomposes to CO2 and the strong base tert-butoxide.
References
For review on synthesis of diazonamide A, see: Lachia, M.; Moody, C. Nat. Prod. Rep. 2008, 25, 227.
Cruz-Monserrate, Z.; Vervoort, H. C.; Bai, R.; Newman, D. J.; Howell, S. B.; Los, G.; Mullaney, J. T.; Williams, M. D.; Pettit, G. R.; Fenical, W.; Hamel, E. Mol. Pharmacol. 2003, 63, 1273.
For review, see: Beaud, R.; Tomakinian, T.; Denizot, N.; Pouilhès, A.; Kouklovsky, C.; Vincent, G. Synlett 2015, 26, 432.
(a) Tomakinian, T.; Kouklovsky, C.; Vincent, G. Synlett 2015, 26, 1269. (b) Ding, H.; DeRoy, P. L.; Perreault, C.; Larivée, A.; Siddiqui, A.; Caldwell, C. G.; Harran, S.; Harran, P. G. Angew. Chem., Int. Ed. 2015, 54, 4818. (c) Tomakinian, T.; Guillot, R.; Kouklovsky, C.; Vincent, G. Angew. Chem., Int. Ed. 2014, 53, 11881. (d) Liao, L.; Shu, C.; Zhang, M.; Liao, Y.; Hu, X.; Zhang, Y.; Wu, Z.; Yuan, W.; Zhang, X. Angew. Chem., Int. Ed. 2014, 53, 10471. (e) Shu, C.; Liao L.-H.; Liao Y.-J.; Hu, X.-Y.; Zhang, Y.-H.; Yuan, W.-C.; Zhang, X.-M. Eur. J. Org. Chem. 2014, 4467. (f) Denizot, N.; Pouilhès, A.; Cucca, M.; Beaud, R.; Guillot, R.; Kouklovsky, C.; Vincent, G. Org. Lett. 2014, 16, 5752. (g) Zhao, J.-C.; Yu, S.-M.; Liu, Y.; Yao, Z.-J. Org. Lett. 2013, 15, 4300. (h) Beaud, R.; Guillot, R.; Kouklovsky, C.; Vincent, G. Angew. Chem., Int. Ed. 2012, 51, 12546.
(a) Zajac, M. A.; Vedejs, E. Org. Lett. 2004, 6, 237. (b) Peris, G.; Vedejs, E. J. Org. Chem. 2015, 80, 3050.
Poriel, C.; Lachia, M.; Wilson, C.; Davies, J. R.; Moody, C. J. J. Org. Chem. 2007, 72, 2978.
Mutule, I.; Joo, B.; Medne, Z.; Kalnins, T.; Vedejs, E.; Suna, E. J. Org. Chem. 2015, 80, 3058.
Rawal, V. H.; Cava, M. P. Tetrahedron Lett. 1985, 26, 6141.
Basel, Y.; Hassner, A. J. Org. Chem. 2000, 65, 6368.
We thank European Social Fund (project No. 1DP/1.1.1.2.0/13/APIA/VIAA/006) for financial support of this research. E. Vedejs thanks InnovaBalt project for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(7), 613–620
Rights and permissions
About this article
Cite this article
Mutule, I., Kalnins, T., Vedejs, E. et al. Diazonamide synthetic studies. Reactivity of N-unsubstituted benzofuro[2,3-b]indolines. Chem Heterocycl Comp 51, 613–620 (2015). https://doi.org/10.1007/s10593-015-1749-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1749-7