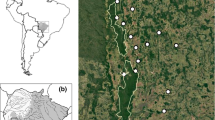
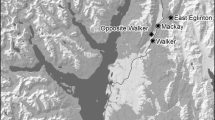
Abstract
Bats are often considered highly mobile and hence less susceptible to habitat fragmentation than other animals. We tested this basic assumption by studying populations of Dermanura watsoni, a frugivorous phyllostomid bat, inhabiting forest fragments in an agriculturally dominated landscape in northeastern Costa Rica. We used the mitochondrial D-loop DNA-sequence data to examine genetic diversity and population structure. A significant population differentiation (F ST = 0.05, p < 0.001) over a geographical scale of approximately 20 km was detected. Genetic diversity within fragments correlated with patch size and the amount of suitable habitat in the surrounding matrix. The composition of the matrix in close proximity to the fragments explained variation in genetic diversity best. However, only habitat parameters measured from 1986 land cover conditions can explain current genetic diversity, and not those from 2001. Our study demonstrates that bats, despite their high mobility, are not secure from genetic erosion in anthropogenically modified landscapes. Population differentiation can occur on a surprisingly small geographic scale and after short time periods. Our findings illustrate the importance of considering several points in time when testing for an influence of habitat parameters as it might be decades until they are reflected by genetic diversity.

Similar content being viewed by others
References
Albrecht L, Meyer CFJ, Kalko EKV (2007) Differential mobility in two small phyllostomid bats, Artibeus watsoni and Micronycteris microtis, in a fragmented neotropical landscape. Acta Theriol 52:141–149
Asher C (2009) Patterns of genetic diversity in populations of two bat species (Sturnira ludovici and Artibeus toltecus) in Cusuco National Park, Honduras. Biosci Horiz 2:147–154
Burland TM, Wilmer JW (2001) Seeing in the dark: molecular approaches to the study of bat populations. Biol Rev 76:389–409
Burland TM, Barratt EM, Beaumont MA, Racey PA (1999) Population genetic structure and gene flow in a gleaning bat, Plecotus auritus. Proc R Soc B 266:975–980
Campbell S, Guay P-J, Mitrovski PJ, Mulder R (2009) Genetic differentiation among populations of a specialist fishing bat suggests lack of suitable habitat connectivity. Biol Conserv 142:2657–2664
Carstens BC, Sullivan J, Davalos LM, Larsen PA, Pedersen SC (2004) Exploring population genetic structure in three species of lesser antillean bats. Mol Ecol 13:2557–2566
Chaverri G, Quirós OE, Kunz TH (2007) Ecological correlates of range size in the tent-making bat Artibeus watsoni. J Mammal 88:477–486
Chaverri G, Schneider CJ, Kunz TH (2008) Mating system of the tent-making bat Artibeus watsoni (Chiroptera: Phyllostomidae). J Mammal 89:1361–1371
Cosson JF, Pons JM, Masson D (1999) Effects of forest fragmentation on frugivorous and nectarivorous bats in French Guiana. J Trop Ecol 15:515–534
Craul M, Chikhi L, Sousa V, Olivieri GL, Rabesandratana A, Zimmermann E, Radespiel U (2009) Influence of forest fragmentation on an endangered large-bodied lemur in northwestern Madagascar. Biol Conserv 142:2862–2871
Cronin JT (2003) Matrix heterogeneity and host-parasitoid interactions in space. Ecology 84:1506–1516
Cunningham M, Moritz C (1998) Genetic effects of forest fragmentation on a rainforest restricted lizard (Scincidae: Gnypetoscincus queenslandiae). Biol Conserv 83:19–30
Daily GC, Ceballos G, Pacheco J, Suzán G, Sánchez-Azofeifa A (2003) Countryside biogeography of neotropical mammals: conservation opportunities in agricultural landscapes of Costa Rica. Conserv Biol 17:1814–1826
de la Peña-Cuéllar E, Stoner K, Avila-Cabadilla L, Martínez-Ramos M, Estrada A (2012) Phyllostomid bat assemblages in different successional stages of tropical rain forest in Chiapas, Mexico. Biodivers Conserv 21:1381–1397
Dhuyvetter H, Gaublomme E, Verdyck P, Desender K (2005) Genetic differentiation among populations of the salt marsh beetle Pogonus littoralis (Coleoptera: Carabidae): a comparison between Atlantic and Mediterranean populations. J Hered 96:381–387
Ditchfield AD (2000) The comparative phylogeography of Neotropical mammals: patterns of intraspecific mitochondrial DNA variation among bats contrasted to nonvolant small mammals. Mol Ecol 9:1307–1318
Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol Conserv 142:1560–1569
Dotta G, Verdade LM (2011) Medium to large-sized mammals in agricultural landscapes of south-eastern Brazil. Mammalia 75:345–352
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Epps CW, Palsbøll PJ, Wehausen JD, Roderick GK, Ramey RR II, McCullough DR (2005) Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecol Lett 8:1029–1038
Ernest HB, Boyce WM, Bleich VC, May B, Stiver SJ, Torres SG (2003) Genetic structure of mountain lion (Puma concolor) populations in California. Conserv Genet 4:353–366
Ewers RM, Didham RK (2007) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv Biol 21:926–936
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Galindo-González J, Guevara S, Sosa VJ (2000) Bat- and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conserv Biol 14:1693–1703
Gerlach G, Musolf K (2000) Fragmentation of landscape as a cause for genetic subdivision in bank voles. Conserv Biol 14:1066–1074
Ghanem SJ, Voigt CC (2012) Increasing awareness of ecosystem services provided by bats. Brockmann HJ, Roper TJ, Naguib M, Mitani JC, Simmons LW (eds). Adv Stud Behav 44:279–302
Giannini NP, Kalko EKV (2004) Trophic structure in a large assemblage of phyllostomid bats in Panama. Oikos 105:209–220
Gorresen PM, Willig MR (2004) Landscape responses of bats to habitat fragmentation in Atlantic forest of Paraguay. J Mammal 85:688–697
Hansen MC, Stehman SV, Potapov PV (2010) Quantification of global gross forest cover loss. Proc Natl Acad Sci USA 107:8650–8655
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Henry M, Pons J-M, Cosson J-F (2007) Foraging behaviour of a frugivorous bat helps bridge landscape connectivity and ecological processes in a fragmented rainforest. J Anim Ecol 3:801–813
Hirota T, Hirohata T, Mashima H, Satoh T, Obara Y (2004) Population structure of the large Japanese field mouse, Apodemus speciosus (Rodentia: Muridae), in suburban landscape, based on mitochondrial D-loop sequences. Mol Ecol 13:3275–3282
Johansson M, Primmer CR, Merilä J (2007) Does habitat fragmentation reduce fitness and adaptability? A case study of the common frog (Rana temporaria). Mol Ecol 16:2693–2700
Kalcounis MC, Hobson KA, Brigham RM, Hecker KR (1999) Bat activity in the boreal forest: importance of stand type and vertical strata. J Mammal 80:673–682
Kerth G, Melber M (2009) Species-specific barrier effects of a motorway on the habitat use of two threatened forest-living bat species. Biol Conserv 142:270–279
Keyghobadi N, Roland J, Matter SF, Strobeck C (2005) Among and within-patch components of genetic diversity respond at different rates to habitat fragmentation: an empirical demonstration. Proc R Soc B 272:553–560
Laurance WF, Albernaz AKM, Da Costa C (2001) Is deforestation accelerating in the Brazilian Amazon? Environ Conserv 28:305–311
Leidner AK, Haddad NM, Lovejoy TE (2010) Does tropical forest fragmentation increase long-term variability of butterfly communities? PLoS ONE 5:e9534
Lobova TA, Cullen K, Mori S (2009) Seed dispersal by bats in the Neotropics. Botanical Garden, New York
Makeeva VM, Belokon MM, Malyuchenko OP, Leont’eva OA (2006) Evaluation of the state of the gene pool of natural populations dwelling in the fragmented landscape of Moscow and Moscow region (with special reference to brown frogs). Russ J Genet 42:505–517
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mello MAR, Marquitti FMD, Guimarães PR, Kalko EKV, Jordano P, Martinez de Aguiar MA (2011) The missing part of seed dispersal networks: structure and robustness of bat–fruit interactions. PLoS ONE 6:e17395
Melo FPL, Rodriguez-Herrera B, Chazdon RL, Medellin RA, Ceballos GG (2009) Small tent-roosting bats promote dispersal of large-seeded plants in a Neotropical forest. Biotropica 41:737–743
Meyer CFJ, Fründ J, Lizano WP, Kalko EKV (2008) Ecological correlates of vulnerability to fragmentation in Neotropical bats. J Appl Ecol 45:381–391
Meyer CFJ, Kalko EKV, Kerth G (2009) Small-scale fragmentation effects on local genetic diversity in two phyllostomid bats with different dispersal abilities in Panama. Biotropica 41:95–102
Müllenbach R, Lagoda PLJ, Welter C (1989) An efficient salt–chloroform extraction of DNA from blood and tissues. Trends Genet 5:391
Muscarella R, Fleming TH (2007) The role of frugivorous bats in Tropical forest succession. Biol Rev 82:573–590
Neuwald JL (2010) Population isolation exacerbates conservation genetic concerns in the endangered Amargosa vole, Microtus californicus scirpensis. Biol Conserv 143:2028–2038
Newton LR, Nassar JM, Fleming TH (2003) Genetic population structure and mobility of two nectar-feeding bats from Venezuelan deserts: inferences from mitochondrial DNA. Mol Ecol 12:3191–3198
Otto SP, Whitlock MC (1997) The probability of fixation in populations of changing size. Genetics 146:723–733
Potter S, Eldridge M, Cooper S, Paplinska J, Taggart D (2012) Habitat connectivity, more than species’ biology, influences genetic differentiation in a habitat specialist, the short-eared rock-wallaby (Petrogale brachyotis). Conserv Genet 13:937–952
Reid FA (2009) A field guide to the mammals of central America & southeast Mexico, 2nd edn. Oxford University Press, New York
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 15:887–899
Riley SPD, Pollinger JP, Sauvajot RM, York EC, Bromley C, Fuller TK, Wayne RK (2006) A southern California freeway is a physical and social barrier to gene flow in carnivores. Mol Ecol 15:1733–1741
Roberts TE (2006) History, ocean channels, and distance determine phylogeographic patterns in three widespread Philippine fruit bats (Pteropodidae). Mol Ecol 15:2183–2199
Roberts D, Baker J, Perrin C (2011) Population genetic structure of the endangered Eastern Bristlebird, Dasyornis brachypterus; implications for conservation. Conserv Genet 12:1075–1085
Rodríguez-Herrera B, Medellín RA, Timm RM (2007) Neotropical tent-roosting bats. INBio, Santo Domingo de Heredia
Rossiter SJ, Zubaid A, Mohd-Adnan A, Struebig MJ, Kunz TH, Gopal S, Petit EJ, Kingston T (2012) Social organization and genetic structure: insights from co-distributed bat populations. Mol Ecol 21:647–661
Sala OE, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sánchez-Azofeifa GA, Pfaff A, Robalino JA, Boomhower JP (2007) Costa Rica’s payment for environmental services program: intention, implementation, and impact. Conserv Biol 21:1165–1173
Schaub A, Ostwald J, Siemers BM (2008) Foraging bats avoid noise. J Exp Biol 211:3174–3180
Sesnie SE, Gessler PE, Finegan B, Thessler S (2008) Integrating landsat TM and SRTM-DEM derived variables with decision trees for habitat classification and change detection in complex neotropical environments. Remote Sens Environ 112:2145–2159
Silveira M, Trevelin L, Port-Carvalho M, Godoi S, Mandetta EN, Cruz-Neto AP (2011) Frugivory by phyllostomid bats (Mammalia: Chiroptera) in a restored area in southeast Brazil. Acta Oecol 37:31
Stiles F, Skutch A (1989) A field guide to the birds of Costa Rica. Cornell University Press, New York
Stow AJ, Briscoe DA (2005) Impact of habitat fragmentation on allelic diversity at microsatellite loci in Cunningham’s skink (Egernia cunninghami); a preliminary study. Conserv Genet 6:455–459
Struebig MJ, Kingston T, Petit EJ, Le Comber SC, Zubaid A, Mohd-Adnan A, Rossiter SJ (2011) Parallel declines in species and genetic diversity in tropical forest fragments. Ecol Lett 14:582–590
Swihart RK, Lusk JJ, Duchamp JE, Rizkalla CE, Moore JE (2006) The roles of landscape context, niche breadth, and range boundaries in predicting species responses to habitat alteration. Divers Distrib 12:277–287
Timm RM, LaVal RK (1998) A field key to the bats of Costa Rica. Occ Pub Ser U Kans 22:1–30
Traill LW, Brook BW, Frankham RR, Bradshaw CJA (2010) Pragmatic population viability targets in a rapidly changing world. Biol Conserv 143:28–34
Turner IM, Corlett RT (1996) The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol Evol 11:330–333
Vandergast AG, Bohonak AJ, Weissman DB, Fisher RN (2007) Understanding the genetic effects of recent habitat fragmentation in the context of evolutionary history: phylogeography and landscape genetics of a southern California endemic Jerusalem cricket (Orthoptera: Stenopelmatidae: Stenopelmatus). Mol Ecol 16:977–992
Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc R Soc B 275:649–659
Wade TG, Riitters KH, Wickham JD, Jones KB (2003) Distribution and causes of global forest fragmentation. Conserv Ecol 7:7
Watson JEM, Whittaker RJ, Dawson TP (2004) Habitat structure and proximity to forest edge affect the abundance and distribution of forest-dependent birds in tropical coastal forests of southeastern Madagascar. Biol Conserv 120:311–327
Wilkinson GS, Chapman AM (1991) Length and sequence variation in evening bat D-loop mtDNA. Genetics 128:607–617
Wilkinson GS, Fleming TH (1996) Migration and evolution of lesser long-nosed bats Leptonycteris curasoae, inferred from mitochondrial DNA. Mol Ecol 5:329–339
Wilkinson GS, Mayer F, Kerth G, Petri B (1997) Evolution of repeated sequence arrays in the D-loop region of bat mitochondrial DNA. Genetics 146:1035–1048
Wright S (1951) The genetical structure of populations. Ann Eugen 15:323–354
Wright SJ, Hernandéz A, Condit R (2007) The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica 39:363–371
Zurcher AA, Sparks DW, Bennett VJ (2010) Why the bat did not cross the road? Acta Chiropterol 12:337–340
Acknowledgments
We thank Alberto Quintana of Hacienda Pozo Azul and Giovanna Holbrook of Selva Verde Lodge for the permission to conduct fieldwork. Thanks to all private land owners for granting access to their properties. For assistance in the field we are grateful to Emanuel Rojas, Elder Miranda, and Katrin Heer, to Martina Nagy for help in the laboratory and Mirjam Knörnschild for statistical advising. We thank Steven Sesnie for providing the land cover maps. Logistical support was provided by Chiquita Brands International. This work was approved by Javier Guevara (Resolutions: 047-2010-SINAC, 004-2011-SINAC, 128-2011-SINAC). Funding for field work was provided by a grant of the “Deutscher Akademischer Austauschdienst” (DAAD).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ripperger, S.P., Tschapka, M., Kalko, E.K.V. et al. Life in a mosaic landscape: anthropogenic habitat fragmentation affects genetic population structure in a frugivorous bat species. Conserv Genet 14, 925–934 (2013). https://doi.org/10.1007/s10592-012-0434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-012-0434-y