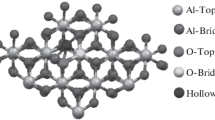
Abstract
Temperature programmed desorption (TPD) of ethanol, as well as ethanol and methanol dehydration reactions were studied on γ-Al2O3 in order to identify the active catalytic sites for alcohol dehydration reactions. Two high temperature (>473 K) desorption features were observed following ethanol adsorption. Samples calcined at T ≤ 473 K displayed a desorption feature in the 523–533 K temperature range, while those calcined at T ≥ 673 K showed a single desorption feature at 498 K. These two high temperature desorption features correspond to the exclusive formation of ethylene on the Lewis (498 K) and Brønsted acidic (~525 K) sites. The amount of ethylene formed under conditions where the competition between water and ethanol for adsorption sites is minimized is identical over the two surfaces. Furthermore, a nearly 1-to-1 correlation between the number of under-coordinated Al3+ ions on the (100) facets of γ-Al2O3 and the number of ethylene molecules formed in the ethanol TPD experiments on samples calcined at T ≥ 673 K was found. Titration of the penta-coordinate Al3+ sites on the (100) facets of γ-Al2O3 by BaO completely eliminated the methanol dehydration reaction activity. These results demonstrate that in alcohol dehydration reactions on γ-Al2O3, the (100) facets are the active catalytic surfaces. The observed activities can be linked to the same Al3+ ions on both hydrated and dehydrated surfaces: penta-coordinate Al3+ ions (Lewis acid sites), and their corresponding –OH groups (Brønsted acid sites), depending on the calcination temperature.
Graphical Abstract
Temperature-programmed desorption of ethanol, as well as steady state dehydration reactions of ethanol and methanol, indicate that the (100) facets are the primary active surfaces of γ-Al2O3. The active centers on both the hydroxylated and dehydroxylated (100) facets are related to the coordinatively unsaturated Al3+ ions





Similar content being viewed by others
References
Knözinger H, Shübner B (1978) J Phys Chem 82:1526
Jeziorowski H, Knözinger H, Meyer W, Müller HD (1973) J Chem Soc Faraday Trans 69:1744
De Canio EC, Nero VP, Bruno JW (1992) J Catal 135:444
Liu X (2008) J Phys Chem C 112:5066
Digne M, Sautet P, Raybaud P, Euzen P, Toulhoat H (2004) J Catal 226:54
Digne M, Sautet P, Raybaud P, Euzen P, Toulhoat H (2002) J Catal 211:1
Onfroy T, Li WC, Shüth F, Knözinger H (2009) Phys Chem Chem Phys 11:3671
Feng G, Huo CF, Deng CM, Huang L, Li YW, Wang J, Jiao J (2009) J Mol Catal A 304:58
Kim S, Sorescu DC, Byl O, Yates JT Jr (2006) J Phys Chem B 220:4742
Kwak JH, Hu JZ, Kim DH, Szanyi J, Peden CHF (2007) J Catal 251:189
Kwak JH, Mei D, Yi CW, Kim DH, Peden CHF, Allard LF, Szanyi J (2009) J Catal 261:17
Kwak JH, Hu JZ, Mei D, Yi CW, Kim DH, Peden CHF, Allard LF, Szanyi J (2009) Science 325:1670–1673
Mei D, Kwak JH, Hu J, Cho SJ, Szanyi J, Allard LF, Peden CHF (2010) J Phys Chem C 1:2688–2691
Szanyi J, Kwak JH, Kim DH, Wang X, Chimentao R, Hanson J, Epling WS, Peden CHF (2007) J Phys Chem C 111:4678
Acknowledgments
We gratefully acknowledge the US Department of Energy (DOE), Office of Basic Energy Sciences, Division of Chemical Sciences for the support of this work. The research described in this paper was performed at the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated for the US DOE by Battelle Memorial Institute under contract number DE-AC05-76RL01830.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kwak, J.H., Mei, D., Peden, C.H.F. et al. (100) facets of γ-Al2O3: The Active Surfaces for Alcohol Dehydration Reactions. Catal Lett 141, 649–655 (2011). https://doi.org/10.1007/s10562-010-0496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0496-8