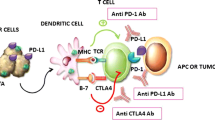
Abstract
Breast cancer is a systemic disease with a primarily local component. Besides surgical resection and irradiation of the locoregional tumor setting, central therapeutic aim is the elimination of disseminated micrometastatic tumor cells using cytostatic and/or hormonal treatment. Nevertheless, in the course of time a majority of patients suffer from systemic recurrence in the form of distant metastases. Intriguingly, in this connection, intratumoral cytotoxic T lymphocytes might serve as independent predictors of treatment efficacy and clinical outcome. Loss of immune balance (tumor dormancy) during intensive cross talk between T cells and tumor cells in the bone marrow microenvironment is suggested one reason for distant metastatic relapse. In this clinical context, further supportive therapies become increasingly attractive, taking immunological features of breast cancer cells into special account. The present review aims to dissect bone marrow-derived cellular antitumor immune responses and translational immunologic treatment options regarding their actual relevance to patients’ clinical benefit and their future directions in breast cancer management.



Similar content being viewed by others
References
Demicheli, R. (2001). Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Seminars in Cancer Biology, 11(4), 297–306.
Mansi, J. L., Gogas, H., Bliss, J. M., Gazet, J. C., Berger, U., & Coombes, R. C. (1999). Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet, 354(9174), 197–202.
Cote, R. J., Rosen, P. P., Lesser, M. L., Old, L. J., & Osborne, M. P. (1991). Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. Journal of Clinical Oncology, 9(10), 1749–1756.
Braun, S., & Naume, B. (2005). Circulating and disseminated tumor cells. Journal of Clinical Oncology, 23(8), 1623–1626.
Braun, S., Vogl, F. D., Naume, B., Janni, W., Osborne, M. P., Coombes, R. C., et al. (2005). A pooled analysis of bone marrow micrometastasis in breast cancer. The New England Journal of Medicine, 353(8), 793–802.
Diel, I. J., Kaufmann, M., Costa, S. D., Holle, R., Von Minckwitz, G., Solomayer, E. F., et al. (1996). Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. Journal of the National Cancer Institute, 88(22), 1652–1658.
Domschke, C., Neubrech, F., Dick, M., Rom, J., Beckhove, P., Sohn, C., et al. (2011). Intraoperative bone marrow puncture in breast cancer patients: prospective assessment of adverse side-effects. Breast, 20(1), 62–65.
Domschke, C., Diel, I. J., Englert, S., Kalteisen, S., Mayer, L., Rom, J., et al. (2012). Prognostic value of disseminated tumor cells in the bone marrow of patients with operable primary breast cancer: a long-term follow-up study. Annals of Surgical Oncology, 20(6), 1865–1871.
Pagès, F., Galon, J., Dieu-Nosjean, M.-C., Tartour, E., Sautès-Fridman, C., & Fridman, W.-H. (2010). Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene, 29(8), 1093–1102.
Calabrò, A., Beissbarth, T., Kuner, R., Stojanov, M., Benner, A., Asslaber, M., et al. (2009). Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Research and Treatment, 116(1), 69–77.
Rody, A., Holtrich, U., Pusztai, L., Liedtke, C., Gaetje, R., Ruckhaeberle, E., et al. (2009). T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Research, 11(2), R15.
Mahmoud, S. M., Paish, E. C., Powe, D. G., Macmillan, R. D., Grainge, M. J., Lee, A. H., et al. (2011). Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of Clinical Oncology, 29(15), 1949–1955.
Liu, S., Lachapelle, J., Leung, S., Gao, D., Foulkes, W. D., & Nielsen, T. O. (2012). CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Research, 14(2), R48.
Yan, M., Jene, N., Byrne, D., Millar, E. K. A., O’Toole, S. A., McNeil, C. M., et al. (2011). Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Research, 13(2), R47.
Mahmoud, S. M. A., Paish, E. C., Powe, D. G., Macmillan, R. D., Lee, A. H. S., Ellis, I. O., et al. (2011). An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Research and Treatment, 127(1), 99–108.
Liu, F., Lang, R., Zhao, J., Zhang, X., Pringle, G. A., Fan, Y., et al. (2011). CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Research and Treatment, 130(2), 645–655.
De Leeuw, R. J., Kost, S. E., Kakal, J. A., & Nelson, B. H. (2012). The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clinical Cancer Research, 18(11), 3022–3029.
Liu, F., Li, Y., Ren, M., Zhang, X., Guo, X., Lang, R., et al. (2012). Peritumoral FOXP3+ regulatory T cell is sensitive to chemotherapy while intratumoral FOXP3+ regulatory T cell is prognostic predictor of breast cancer patients. Breast Cancer Research and Treatment, 135(2), 459–467.
Ma, C., Zhang, Q., Ye, J., Wang, F., Zhang, Y., Wevers, E., et al. (2012). Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. Journal of Immunology, 189(10), 5029–5036.
Ye, J., Ma, C., Wang, F., Hsueh, E. C., Toth, K., Huang, Y., et al. (2013). Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer Research, 73(20), 6137–6148.
Bos, R., & Sherman, L. A. (2010). CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Research, 70(21), 8368–8377.
Bos, R., Marquardt, K. L., Cheung, J., & Sherman, L. A. (2012). Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology, 1(8), 1239–1247.
Gu-Trantien, C., Loi, S., Garaud, S., Equeter, C., Libin, M., De Wind, A., et al. (2013). CD4+ follicular helper T cell infiltration predicts breast cancer survival. The Journal of Clinical Investigation, 123(7), 2873–2892.
Cimino-Mathews, A., Ye, X., Meeker, A., Argani, P., & Emens, L. A. (2013). Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Human Pathology, 44(10), 2055–2063.
Ono, M., Tsuda, H., Shimizu, C., Yamamoto, S., Shibata, T., Yamamoto, H., et al. (2012). Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Research and Treatment, 132(3), 793–805.
Lee, H. J., Seo, J.-Y., Ahn, J.-H., Ahn, S.-H., & Gong, G. (2013). Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. Journal of Breast Cancer, 16(1), 32–39.
Denkert, C., Loibl, S., Noske, A., Roller, M., Müller, B. M., Komor, M., et al. (2010). Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of Clinical Oncology, 28(1), 105–113.
West, N. R., Milne, K., Truong, P. T., Macpherson, N., Nelson, B. H., & Watson, P. H. (2011). Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Research, 13(6), R126.
Loi, S., Sirtaine, N., Piette, F., Salgado, R., Viale, G., Van Eenoo, F., et al. (2013). Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. Journal of Clinical Oncology, 31(7), 860–867.
Andre, F., Dieci, M. V., Dubsky, P., Sotiriou, C., Curigliano, G., Denkert, C., et al. (2013). Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clinical Cancer Research, 19(1), 28–33.
Song, G., Wang, X., Jia, J., Yuan, Y., Wan, F., Zhou, X., et al. (2013). Elevated level of peripheral CD8(+)CD28(−) T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapy. Cancer Immunology, Immunotherapy, 62(6), 1123–1130.
Loi, S. (2013). Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology, 2(7), e24720.
Chan, M. S. M., Wang, L., Felizola, S. J. A., Ueno, T., Toi, M., Loo, W., et al. (2012). Changes of tumor infiltrating lymphocyte subtypes before and after neoadjuvant endocrine therapy in estrogen receptor-positive breast cancer patients–an immunohistochemical study of Cd8+ and Foxp3+ using double immunostaining with correlation to the path. The International Journal of Biological Markers, 27(4), e295–e304.
Zitvogel, L., Apetoh, L., Ghiringhelli, F., & Kroemer, G. (2008). Immunological aspects of cancer chemotherapy. Nature Reviews. Immunology, 8(1), 59–73.
Casares, N., Pequignot, M. O., Tesniere, A., Ghiringhelli, F., Roux, S., Chaput, N., et al. (2005). Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. The Journal of Experimental Medicine, 202(12), 1691–1701.
Obeid, M., Tesniere, A., Ghiringhelli, F., Fimia, G. M., Apetoh, L., Perfettini, J. L., et al. (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine, 13(1), 54–61.
Disis, M. L., Bernhard, H., & Jaffee, E. M. (2009). Use of tumour-responsive T cells as cancer treatment. Lancet, 373(9664), 673–683.
Osmond, D. G. (1994). Production and selection of B lymphocytes in bone marrow: lymphostromal interactions and apoptosis in normal, mutant and transgenic mice. Advances in Experimental Medicine and Biology, 355, 15–20.
Stefanovic, S., Schuetz, F., Sohn, C., Beckhove, P., & Domschke, C. (2013). Bone marrow microenvironment in cancer patients: immunological aspects and clinical implications. Cancer Metastasis Reviews, 32(1–2), 163–178.
Feuerer, M., Beckhove, P., Garbi, N., Mahnke, Y., Limmer, A., Hommel, M., et al. (2003). Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nature Medicine, 9(9), 1151–1157.
Schirrmacher, V., Feuerer, M., Fournier, P., Ahlert, T., Umansky, V., & Beckhove, P. (2003). T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends in Molecular Medicine, 9(12), 526–534.
Mazo, I. B., Honczarenko, M., Leung, H., Cavanagh, L. L., Bonasio, R., Weninger, W., et al. (2005). Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity, 22(2), 259–270.
Feuerer, M., Beckhove, P., Mahnke, Y., Hommel, M., Kyewski, B., Hamann, A., et al. (2004). Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. International Journal of Oncology, 25(4), 867–876.
Khazaie, K., Prifti, S., Beckhove, P., Griesbach, A., Russell, S., Collins, M., et al. (1994). Persistence of dormant tumor cells in the bone marrow of tumor cell-vaccinated mice correlates with long-term immunological protection. Proceedings of the National Academy of Sciences of the United States of America, 91(16), 7430–7434.
Schirrmacher, V., Feuerer, M., Beckhove, P., Ahlert, T., & Umansky, V. (2002). T cell memory, anergy and immunotherapy in breast cancer. Journal of Mammary Gland Biology and Neoplasia, 7(2), 201–208.
Mahnke, Y. D., Schwendemann, J., Beckhove, P., & Schirrmacher, V. (2005). Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells. Immunology, 115(3), 325–336.
Müller, M., Gounari, F., Prifti, S., Hacker, H. J., Schirrmacher, V., & Khazaie, K. (1998). EblacZ tumor dormancy in bone marrow and lymph nodes: active control of proliferating tumor cells by CD8+ immune T cells. Cancer Research, 58(23), 5439–5446.
Bai, L., Beckhove, P., Feuerer, M., Umansky, V., Choi, C., Solomayer, F. S. E.-F., et al. (2003). Cognate interactions between memory T cells and tumor antigen-presenting dendritic cells from bone marrow of breast cancer patients: bidirectional cell stimulation, survival and antitumor activity in vivo. International Journal of Cancer, 103(1), 73–83.
Goldrath, A. W., & Bevan, M. J. (1999). Selecting and maintaining a diverse T-cell repertoire. Nature, 402(6759), 255–262.
Lanzavecchia, A., & Sallusto, F. (2000). From synapses to immunological memory: the role of sustained T cell stimulation. Current Opinion in Immunology, 12(1), 92–98.
Zinkernagel, R. M., Bachmann, M. F., Kündig, T. M., Oehen, S., Pirchet, H., & Hengartner, H. (1996). On immunological memory. Annual Review of Immunology, 14, 333–367.
Schwendemann, J., Choi, C., Schirrmacher, V., & Beckhove, P. (2005). Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. Journal of Immunology, 175(3), 1433–1439.
Hamann, D., Baars, P. A., Rep, M. H., Hooibrink, B., Kerkhof-Garde, S. R., Klein, M. R., et al. (1997). Phenotypic and functional separation of memory and effector human CD8+ T cells. The Journal of Experimental Medicine, 186(9), 1407–1418.
Sallusto, F., Lenig, D., Förster, R., Lipp, M., & Lanzavecchia, A. (1999). Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature, 401(6754), 708–712.
Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A., & Rocha, B. (2000). Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nature Immunology, 1(1), 47–53.
Choi, C., Witzens, M., Bucur, M., Feuerer, M., Sommerfeldt, N., Trojan, A., et al. (2005). Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood, 105(5), 2132–2134.
Feuerer, M., Beckhove, P., Bai, L., Solomayer, E. F., Bastert, G., Diel, I. J., et al. (2001). Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nature Medicine, 7(4), 452–458.
Müller-Berghaus, J., Ehlert, K., Ugurel, S., Umansky, V., Bucur, M., Schirrmacher, V., et al. (2006). Melanoma-reactive T cells in the bone marrow of melanoma patients: association with disease stage and disease duration. Cancer Research, 66(12), 5997–6001.
Sommerfeldt, N., Schütz, F., Sohn, C., Förster, J., Schirrmacher, V., & Beckhove, P. (2006). The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Research, 66(16), 8258–8265.
Solomayer, E.-F., Feuerer, M., Bai, L., Umansky, V., Beckhove, P., Meyberg, G. C., et al. (2003). Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clinical Cancer Research, 9(1), 174–180.
Feuerer, M., Rocha, M., Bai, L., Umansky, V., Solomayer, E. F., Bastert, G., et al. (2001). Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. International Journal of Cancer, 92(1), 96–105.
Kämmerer, U., Thanner, F., Kapp, M., Dietl, J., & Sütterlin, M. (2003). Expression of tumor markers on breast and ovarian cancer cell lines. Anticancer Research, 23(2A), 1051–1055.
Jiang, X. P., Yang, D. C., Elliott, R. L., & Head, J. F. (2000). Vaccination with a mixed vaccine of autogenous and allogeneic breast cancer cells and tumor associated antigens CA15–3, CEA and CA125—results in immune and clinical responses in breast cancer patients. Cancer Biotherapy & Radiopharmaceuticals, 15(5), 495–505.
Bai, L., Feuerer, M., Beckhove, P., Umansky, V., & Schirrmacher, V. (2002). Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. International Journal of Oncology, 20(2), 247–253.
Beckhove, P., Feuerer, M., Dolenc, M., Schuetz, F., Choi, C., Sommerfeldt, N., et al. (2004). Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. The Journal of Clinical Investigation, 114(1), 67–76.
Schuetz, F., Ehlert, K., Ge, Y., Schneeweiss, A., Rom, J., Inzkirweli, N., et al. (2009). Treatment of advanced metastasized breast cancer with bone marrow-derived tumour-reactive memory T cells: a pilot clinical study. Cancer Immunology, Immunotherapy, 58(6), 887–900.
Yee, C., Thompson, J. A., Roche, P., Byrd, D. R., Lee, P. P., Piepkorn, M., et al. (2000). Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. The Journal of Experimental Medicine, 192(11), 1637–1644.
Dudley, M. E., Wunderlich, J. R., Yang, J. C., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., et al. (2002). A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. Journal of Immunotherapy, 25(3), 243–251.
Domschke, C., Ge, Y., Bernhardt, I., Schott, S., Keim, S., Juenger, S., et al. (2013). Long-term survival after adoptive bone marrow T cell therapy of advanced metastasized breast cancer: follow-up analysis of a clinical pilot trial. Cancer Immunology, Immunotherapy, 62(6), 1053–1060.
Chirgwin, J. M., & Guise, T. A. (2000). Molecular mechanisms of tumor–bone interactions in osteolytic metastases. Critical Reviews in Eukaryotic Gene Expression, 10(2), 159–178.
Domschke, C., Schuetz, F., Ge, Y., Seibel, T., Falk, C., Brors, B., et al. (2009). Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Research, 69(21), 8420–8428.
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A.-K. L., & Flavell, R. A. (2006). Transforming growth factor-beta regulation of immune responses. Annual Review of Immunology, 24, 99–146.
Chen, W., Jin, W., Hardegen, N., Lei, K.-J., Li, L., Marinos, N., et al. (2003). Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of Experimental Medicine, 198(12), 1875–1886.
Saad, E. D., Katz, A., & Buyse, M. (2010). Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. Journal of Clinical Oncology, 28(11), 1958–1962.
Schreiber, R. D., Old, L. J., & Smyth, M. J. (2011). Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science, 331(6024), 1565–1570.
Vesely, M. D., Kershaw, M. H., Schreiber, R. D., & Smyth, M. J. (2011). Natural innate and adaptive immunity to cancer. Annual Review of Immunology, 29, 235–271.
Zhou, G., & Levitsky, H. (2012). Towards curative cancer immunotherapy: overcoming posttherapy tumor escape. Clinical & Developmental Immunology, 2012, 124187.
Huehn, J., Siegmund, K., Lehmann, J. C. U., Siewert, C., Haubold, U., Feuerer, M., et al. (2004). Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. The Journal of Experimental Medicine, 199(3), 303–313.
Liu, Z., Kim, J. H., Falo, L. D., & You, Z. (2009). Tumor regulatory T cells potently abrogate antitumor immunity. Journal of Immunology, 182(10), 6160–6167.
Bonertz, A., Weitz, J., Pietsch, D.-H. K., Rahbari, N. N., Schlude, C., Ge, Y., et al. (2009). Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. The Journal of Clinical Investigation, 119(11), 3311–3321.
Nummer, D., Suri-Payer, E., Schmitz-Winnenthal, H., Bonertz, A., Galindo, L., Antolovich, D., et al. (2007). Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. Journal of the National Cancer Institute, 99(15), 1188–1199.
Sakaguchi, S., Wing, K., Onishi, Y., Prieto-Martin, P., & Yamaguchi, T. (2009). Regulatory T cells: how do they suppress immune responses? International Immunology, 21(10), 1105–1111.
Litzinger, M. T., Fernando, R., Curiel, T. J., Grosenbach, D. W., Schlom, J., & Palena, C. (2007). IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood, 110(9), 3192–3201.
Salagianni, M., Lekka, E., Moustaki, A., Iliopoulou, E. G., Baxevanis, C. N., Papamichail, M., et al. (2011). NK cell adoptive transfer combined with Ontak-mediated regulatory T cell elimination induces effective adaptive antitumor immune responses. Journal of Immunology, 186(6), 3327–3335.
Zou, W. (2006). Regulatory T cells, tumour immunity and immunotherapy. Nature reviews. Immunology, 6(4), 295–307.
Ruter, J., Barnett, B. G., Kryczek, I., Brumlik, M. J., Daniel, B. J., Coukos, G., et al. (2009). Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Frontiers in Bioscience, 14, 1761–1770.
Beyer, M., & Schultze, J. L. (2006). Regulatory T cells in cancer. Blood, 108(3), 804–811.
Petrausch, U., Poehlein, C. H., Jensen, S. M., Twitty, C., Thompson, J. A., Assmann, I., et al. (2009). Cancer immunotherapy: the role regulatory T cells play and what can be done to overcome their inhibitory effects. Current Molecular Medicine, 9(6), 673–682.
Ghiringhelli, F., Menard, C., Puig, P. E., Ladoire, S., Roux, S., Martin, F., et al. (2007). Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunology, Immunotherapy, 56(5), 641–648.
Ghiringhelli, F., Larmonier, N., Schmitt, E., Parcellier, A., Cathelin, D., Garrido, C., et al. (2004). CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. European Journal of Immunology, 34(2), 336–344.
Lutsiak, M. E. C., Semnani, R. T., De Pascalis, R., Kashmiri, S. V. S., Schlom, J., & Sabzevari, H. (2005). Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood, 105(7), 2862–2868.
Ge, Y., Domschke, C., Stoiber, N., Schott, S., Heil, J., Rom, J., et al. (2012). Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunology, Immunotherapy, 61(3), 353–362.
Dudley, M. E., Wunderlich, J. R., Robbins, P. F., Yang, J. C., Hwu, P., Schwartzentruber, D. J., et al. (2002). Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science, 298(5594), 850–854.
Oelke, M., Maus, M. V., Didiano, D., June, C. H., Mackensen, A., & Schneck, J. P. (2003). Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nature Medicine, 9(5), 619–624.
Chmielewski, M., & Abken, H. (2012). CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunology, Immunotherapy, 61(8), 1269–1277.
Koehler, P., Schmidt, P., Hombach, A. A., Hallek, M., & Abken, H. (2012). Engineered T cells for the adoptive therapy of B-cell chronic lymphocytic leukaemia. Advances in Hematology, 2012, 595060.
Wu, R., Forget, M.-A., Chacon, J., Bernatchez, C., Haymaker, C., Chen, J. Q., et al. (2012). Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer Journal, 18(2), 160–175.
Ellebaek, E., Iversen, T. Z., Junker, N., Donia, M., Engell-Noerregaard, L., Met, O., et al. (2012). Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose interleukin-2 in metastatic melanoma patients. Journal of Translational Medicine, 10(1), 169.
Radvanyi, L. G., Bernatchez, C., Zhang, M., Fox, P., Miller, P., Chacon, J., et al. (2012). Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clinical Cancer Research, 18(24), 6758–6770.
Besser, M. J., Shapira-Frommer, R., Treves, A. J., Zippel, D., Itzhaki, O., Hershkovitz, L., et al. (2010). Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clinical Cancer Research, 16(9), 2646–2655.
West, N. R., Kost, S. E., Martin, S. D., Milne, K., Deleeuw, R. J., Nelson, B. H., et al. (2013). Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. British Journal of Cancer, 108(1), 155–162.
Oda, N., Shimazu, K., Naoi, Y., Morimoto, K., Shimomura, A., Shimoda, M., et al. (2012). Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Research and Treatment, 136(1), 107–116.
Shi, H., Liu, L., & Wang, Z. (2012). Improving the efficacy and safety of engineered T cell therapy for cancer. Cancer Letters, 328(2), 191–197.
Cordova, A., Toia, F., La Mendola, C., Orlando, V., Meraviglia, S., Rinaldi, G., et al. (2012). Characterization of human γδ T lymphocytes infiltrating primary malignant melanomas. PloS One, 7(11), e49878.
Hadrup, S. R. (2012). The antigen specific composition of melanoma tumor infiltrating lymphocytes? Oncoimmunology, 1(6), 935–936.
Morgan, R. A., Dudley, M. E., Wunderlich, J. R., Hughes, M. S., Yang, J. C., Sherry, R. M., et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science, 314(5796), 126–129.
Johnson, L. A., Morgan, R. A., Dudley, M. E., Cassard, L., Yang, J. C., Hughes, M. S., et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood, 114(3), 535–546.
Robbins, P. F., Morgan, R. A., Feldman, S. A., Yang, J. C., Sherry, R. M., Dudley, M. E., et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of Clinical Oncology, 29(7), 917–924.
Yvon, E., Del Vecchio, M., Savoldo, B., Hoyos, V., Dutour, A., Anichini, A., et al. (2009). Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clinical Cancer Research, 15(18), 5852–5860.
Lo, A. S. Y., Ma, Q., Liu, D. L., & Junghans, R. P. (2010). Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clinical Cancer Research, 16(10), 2769–2780.
Burns, W. R., Zhao, Y., Frankel, T. L., Hinrichs, C. S., Zheng, Z., Xu, H., et al. (2010). A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Research, 70(8), 3027–3033.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanovic, S., Schuetz, F., Sohn, C. et al. Adoptive immunotherapy of metastatic breast cancer: present and future. Cancer Metastasis Rev 33, 309–320 (2014). https://doi.org/10.1007/s10555-013-9452-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9452-6