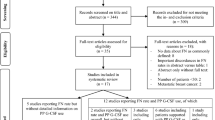
Abstract
Infections related to anti-HER2 monoclonal antibodies (mAbs), trastuzumab and pertuzumab, have been reported in clinical trials. It is not yet clear whether these drugs increase an infection risk or not. We performed a systematic review and meta-analysis to assess the risk of infections associated with anti-HER2 mAbs. We searched PubMed and the ASCO online database of meeting abstracts up to January 2014 for relevant clinical trials. Eligible studies included randomized controlled trials of trastuzumab or pertuzumab for breast cancer patients that reported adequate safety data for grade 3–4 infection, febrile neutropenia, neutropenia, or leukopenia. The summary incidence, relative risk (RR), and 95 % confidence intervals (CIs) were calculated. A total of 10,094 patients from 13 trials were included. The use of trastuzumab was associated with an increased risk of high-grade infection (RR 1.21, 95 % CI 1.07–1.37, P = 0.002) and febrile neutropenia (RR 1.28, 95 % CI 1.08–1.52, P = 0.004). The incidence of high-grade infection and febrile neutropenia due to trastuzumab was 8.5 % (95 % CI 4.5–15.4 %) and 12.0 % (95 % CI 8.1–17.4 %), respectively. There was no significant increase in a risk of high-grade neutropenia or leukopenia in patients receiving trastuzumab. Treatment with trastuzumab is associated with a significantly higher risk of high-grade infection and febrile neutropenia. Our findings suggest an importance of close monitoring for any signs of infections in patients treated with trastuzumab.


Similar content being viewed by others
References
Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM (2003) Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 200(3):290–297
Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9(7):463–475
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr et al (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421(6924):756–760
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4):317–328
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW et al (2011) Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 29(34):4491–4497
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32
Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M et al (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14(6):461–471
Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y et al (2011) Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev 37(4):312–320
Valachis A, Nearchou A, Polyzos NP, Lind P (2013) Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer 133(9):2245–2252
Li H, Fu W, Gao X, Xu Q, Wu H, Tan W (2013) Risk of severe diarrhea with dual anti-HER2 therapies: a meta-analysis. Tumour Biol 35(5):4077–4085
Drucker AM, Wu S, Dang CT, Lacouture ME (2012) Risk of rash with the anti-HER2 dimerization antibody pertuzumab: a meta-analysis. Breast Cancer Res Treat 135(2):347–354
Barni S, Cabiddu M, Guarneri P, Lonati V, Petrelli F (2012) The risk for anemia with targeted therapies for solid tumors. Oncologist 17(5):715–724
Genentech: herceptin prescribing information. http://www.gene.com/download/pdf/herceptin_prescribing.pdf
Genentech: Perjeta prescribing information. http://www.gene.com/download/pdf/perjeta_prescribing.pdf
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
National cancer institute: common toxicity criteria, version 3. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23(19):4265–4274
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R et al (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354(8):809–820
Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G et al (2007) Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat 101(3):355–365
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of cancer and leukemia group B protocol 9840. J Clin Oncol 26(10):1642–1649
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol 27(12):1999–2006
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27(33):5529–5537
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H et al (2010) Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28(12):2024–2031
Pierga JY, Delaloge S, Espie M, Brain E, Sigal-Zafrani B, Mathieu MC et al (2010) A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat 122(2):429–437
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53(11):1119–1129
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V et al (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 18(4):CD006243
Seruga B, Sterling L, Wang L, Tannock IF (2011) Reporting of serious adverse drug reactions of targeted anticancer agents in pivotal phase III clinical trials. J Clin Oncol 29(2):174–185
Acknowledgments
The authors would like to thank Dominic T. Moore for statistical analysis support.
Conflict of interest
Dr. Muss has held consultant/advisory role for Pfizer. The remaining authors state that they have no conflict on interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Funakoshi, T., Suzuki, M. & Muss, H.B. Infection risk in breast cancer patients treated with trastuzumab: a systematic review and meta-analysis. Breast Cancer Res Treat 149, 321–330 (2015). https://doi.org/10.1007/s10549-014-3184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3184-3