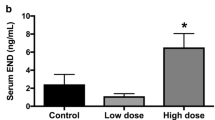
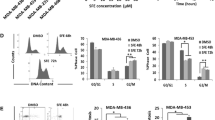
Abstract
Organosulfur compounds from garlic effectively inhibit growth of transplanted as well as spontaneous cancers in preclinical animal models without any adverse side effects. However, the mechanisms underlying anticancer effect of this class of compounds are not fully understood. This study reports, for the first time, that garlic organosulfide diallyl trisulfide (DATS) inhibits estrogen receptor-α (ER-α) activity in human breast cancer cells. Exposure of MCF-7 and T47D cells to DATS resulted in downregulation of ER-α protein, which peaked between 12- and 24-h post-treatment. DATS was relatively more effective in suppressing ER-α protein expression compared with its mono and disulfide analogs. The 17β-estradiol (E2)-induced expression of pS2 and cyclin D1, ER-α target gene products, was also decreased in the presence of DATS. Downregulation of ER-α protein expression resulting from DATS treatment was accompanied by a decrease in nuclear levels of ER-α protein, ER-α mRNA suppression, and inhibition of ERE2e1b-luciferase reporter activity. DATS-mediated inhibition of cell viability and apoptosis induction were not affected in the presence of E2. In agreement with these results, ectopic expression of ER-α in MDA-MB-231 cell line failed to confer any protection against cell proliferation inhibition or apoptosis induction resulting from DATS exposure. DATS treatment caused a decrease in protein levels of peptidyl-prolyl cis–trans isomerase (Pin1), and overexpression of Pin1 partially attenuated ER-α downregulation by DATS. DATS-induced apoptosis was modestly but significantly augmented by overexpression of Pin1. In conclusion, this study identifies ER-α as a novel target of DATS in mammary cancer cells.






Similar content being viewed by others
Abbreviations
- cFBS:
-
Charcoal/dextran-stripped fetal bovine serum
- DAS:
-
Diallyl sulfide
- DADS:
-
Diallyl disulfide
- DATS:
-
Diallyl trisulfide
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
- E2:
-
17β-Estradiol
- ER-α:
-
Estrogen receptor-α
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- PBS:
-
Phosphate-buffered saline
- Pin1:
-
Peptidyl-prolyl cis–trans isomerase
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
References
Rivlin RS (2001) Historical perspective on the use of garlic. J Nutr 131(3):951S–954S
Rahman K (2001) Historical perspective on garlic and cardiovascular disease. J Nutr 131(3):977S–979S
Dausch JG, Nixon DW (1990) Garlic: a review of its relationship to malignant disease. Prev Med 19(3):346–361
Agarwal KC (1996) Therapeutic actions of garlic constituents. Med Res Rev 16(1):111–124
El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS (2006) Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr 136(3):864S–869S
Block E (1992) The organosulfur chemistry of the genus Allium: implications for the organic chemistry of sulfur. Angew Chem Int Ed Engl 31(9):1135–1178
You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF Jr, Wang TG (1989) Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst 81(2):162–164
Dorant E, van den Brandt PA, Goldbohm RA (1995) Allium vegetable consumption, garlic supplement intake, and female breast carcinoma incidence. Breast Cancer Res Treat 33(2):163–170
Challier B, Perarnau JM, Viel JF (1998) Garlic, onion and cereal fiber as protective factors for breast cancer: a French case–control study. Eur J Epidemiol 14(8):737–747
Fleischauer AT, Poole C, Arab L (2000) Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr 72(4):1047–1052
Herman-Antosiewicz A, Powolny AA, Singh SV (2007) Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin 28(9):1355–1364
Antony ML, Singh SV (2011) Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Ind J Exp Biol 49(11):805–816
Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV (2004) Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene 23(33):5594–5606
Na HK, Kim EH, Choi MA, Park JM, Kim DH, Surh YJ (2012) Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem Pharmacol 84(10):1241–1250
Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher RJ, Zaren HA, Awasthi S, Awasthi YC, Singh SV (1997) Induction of glutathione S-transferase π as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer 73(6):897–902
Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV (2006) Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res 12(22):6836–6843
Singh SV, Powolny AA, Stan SD, Xiao D, Arlotti JA, Warin R, Hahm ER, Marynowski SW, Bommareddy A, Potter DM, Dhir R (2008) Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res 68(22):9503–9511
Malki A, El-Saadani M, Sultan AS (2009) Garlic constituent diallyl trisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther 8(22):2175–2185
Nkrumah-Elie YM, Reuben JS, Hudson A, Taka E, Badisa R, Ardley T, Israel B, Sadrud-Din SY, Oriaku E, Darling-Reed SF (2012) Diallyl trisulfide as an inhibitor of benzo(a)pyrene-induced precancerous carcinogenesis in MCF-10A cells. Food Chem Toxicol 50(7):2524–2530
Chandra-Kuntal K, Lee J, Singh SV (2013) Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat 138(1):69–79
Hahm ER, Lee J, Huang Y, Singh SV (2011) Withaferin A suppresses estrogen receptor-α expression in human breast cancer cells. Mol Carcinog 50(8):614–624
Sakao K, Singh SV (2012) d,l-Sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J Cell Biochem 113(2):599–610
Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV (2003) Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 24(5):891–897
Shah YM, Kaul A, Dong Y, Ip C, Rowan BG (2005) Attenuation of estrogen receptor α (ERα) signaling by selenium in breast cancer cells via downregulation of ERα gene expression. Breast Cancer Res Treat 92(3):239–250
Lee BC, Park BH, Kim SY, Lee YJ (2011) Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem 112(1):118–127
Sui M, Huang Y, Park BH, Davidson NE, Fan W (2007) Estrogen receptor α mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res 67(11):5337–5344
Rajbhandari P, Finn G, Solodin NM, Singarapu KK, Sahu SC, Markley JL, Kadunc KJ, Ellison-Zelski SJ, Kariagina A, Haslam SZ, Lu KP, Alarid ET (2012) Regulation of estrogen receptor α N-terminus conformation and function by peptidyl-prolyl isomerase Pin1. Mol Cell Biol 32(2):445–457
Rajbhandari P, Schalper KA, Solodin NM, Ellison-Zelski SJ, Ping LK, Rimm DL, Alarid ET (2013) Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene. doi:10.1038/onc.2013.78
Lucchetti C, Caligiuri I, Toffoli G, Giordano A, Rizzolio F (2013) The prolyl isomerase Pin1 acts synergistically with CDK2 to regulate the basal activity of estrogen receptor α in breast cancer. PLoS ONE 8(2):e55355
Lee TH, Pastorino L, Lu KP (2011) Peptidyl-prolyl cis–trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert Rev Mol Med 13:e21
Spears M, Bartlett J (2009) The potential role of estrogen receptors and the SRC family as targets for the treatment of breast cancer. Expert Opin Ther Targets 13(6):665–674
Welboren WJ, Sweep FC, Span PN, Stunnenberg HG (2009) Genomic actions of estrogen receptor α: what are the targets and how are they regulated? Endocr Relat Cancer 16(4):1073–1089
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388
Obiorah I, Jordan VC (2011) Progress in endocrine approaches to the treatment and prevention of breast cancer. Maturitas 70(4):315–321
Sun X, Guo T, He J, Zhao M, Yan M, Cui F, Deng Y (2006) Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi 126(7):521–527
Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, Sun PH, Xie XY (2004) An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin Med J 117(8):1155–1160
Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144(10):4562–4574
Kim MR, Choi HK, Cho KB, Kim HS, Kang KW (2009) Involvement of Pin1 induction in epithelial–mesenchymal transition of tamoxifen-resistant breast cancer cells. Cancer Sci 100(10):1834–1841
Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, Farrerons J, Karasik A, Mellstrom D, Ng KW, Stepan JJ, Powles TJ, Morrow M, Costa A, Silfen SL, Walls EL, Schmitt H, Muchmore DB, Jordan VC, Ste-Marie LG (2001) Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial: multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat 65(2):125–134
Acknowledgments
The authors thank Dr. Yi Huang (University of Pittsburgh Cancer Institute, Pittsburgh, PA) for generous gift of MDA-MB-231 cells stably transfected with ER-α plasmid and corresponding empty vector transfected control cells.
Conflict of interest
None of the authors has any conflict of interest.
Funding
This investigation was supported by USPHS grant RO1 CA113363-09, awarded by the National Cancer Institute of the National Institutes of Health. This research project used the Flow Cytometry Facility supported in part by the Cancer center Support Grant P30 CA047904 from the National Cancer Institute of the National Institutes of Health. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hahm, ER., Singh, S.V. Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res Treat 144, 47–57 (2014). https://doi.org/10.1007/s10549-014-2841-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2841-x