Abstract
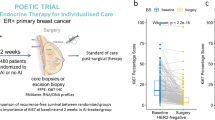
There is growing evidence that uncontrolled activation of the PI3K/Akt/mTOR pathway contributes to the development and progression of breast cancer. Inhibition of this pathway has antitumour effects in preclinical studies and efficacy in combination with other agents in breast cancer patients. The aim of this study is to characterise the effects of pre-operative everolimus treatment in primary breast cancer patients and to identify potential molecular predictors of response. Twenty-seven patients with oestrogen receptor (ER)-positive breast cancer completed 11–14 days of neoadjuvant treatment with 5-mg everolimus. Core biopsies were taken before and after treatment and analysed using Illumina HumanRef-8 v2 Expression BeadChips. Changes in proliferation (Ki67) and phospho-AKT were measured on diagnostic core biopsies/resection samples embedded in paraffin by immunohistochemistry to determine response to treatment. Patients that responded to everolimus treatment with significant reductions in proliferation (fall in % Ki67 positive cells) also had significant decreases in the expression of genes involved in cell cycle (P = 8.70E−09) and p53 signalling (P = 0.01) pathways. Highly proliferating tumours that have a poor prognosis exhibited dramatic reductions in the expression of cell cycle genes following everolimus treatment. The genes that most clearly separated responding from non-responding pre-treatment tumours were those involved with protein modification and dephosphorylation, including DYNLRB2, ERBB4, PTPN13, ULK2 and DUSP16. The majority of ER-positive breast tumours treated with everolimus showed a significant reduction in genes involved with proliferation, these may serve as markers of response and predict which patients will derive most benefit from mTOR inhibition.




Similar content being viewed by others
References
Dillon RL, White DE, Muller WJ (2007) The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26:1338–1345
Liedtke C, Cardone L, Tordai A, Yan K, Gomez HL, Figureoa LJ, Hubbard RE, Valero V, Souchon EA, Symmans WF, Hortobagyi GN, Bardelli A, Pusztai L (2008) PIK3CA-activating mutations and chemotherapy sensitivity in stage II-III breast cancer. Breast Cancer Res 10:R27
Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA (2004) Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 64:7678–7681
Depowski PL, Rosenthal SI, Ross JS (2001) Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol 14:672–676
Perez-Tenorio G, Alkhori L, Olsson B, Waltersson MA, Nordenskjold B, Rutqvist LE, Skoog L, Stal O (2007) PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res 13:3577–3584
Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM (2005) AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207:139–146
Stal O, Perez-Tenorio G, Akerberg L, Olsson B, Nordenskjold B, Skoog L, Rutqvist LE (2003) Akt kinases in breast cancer and the results of adjuvant therapy. Breast Cancer Res 5:R37–R44
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395–402
Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN (2009) The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev 35:148–159
Di Cosimo S, Baselga J (2008) Targeted therapies in breast cancer: where are we now? Eur J Cancer 44:2781–2790
Treeck O, Wackwitz B, Haus U, Ortmann O (2006) Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol 102:292–299
Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, Natt F, Hall J, Lane HA, Thomas G (2005) The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 120:747–759
Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O’Reilly T, Evans DB, Chen S, Lane HA (2005) Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res 11:5319–5328
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610
Awada A, Cardoso F, Fontaine C, Dirix L, De Greve J, Sotiriou C, Steinseifer J, Wouters C, Tanaka C, Zoellner U, Tang P, Piccart M (2008) The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer 44:84–91
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2630–2637
Macaskill EJ, Bartlett JMS, Sabine VS, Faratian D, Renshaw L, White S, Campbell FM, Young O, Williams L, Thomas JS, Barber MD, Dixon JM (submitted) The mammalian target of rapamycin (mTOR) inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res Treat
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’hern R, Salter J, Detre S, Hills M, Walsh G (2007) IMPACT Trialists Group Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170
Jones RL, Salter J, A’hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M (2009) The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 116:53–68
Johnston SR (2008) Integration of endocrine therapy with targeted agents. Breast Cancer Res 10:S20 (suppl 4)
Morgensztern D, McLeod HL (2005) PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 16:797–803
Sims AH, Ong KR, Clarke RB, Howell A (2006) High-throughout genomic technology in research and clinical management of breast cancer: Exploiting the potential of gene expression profiling: is it ready for the clinic? Breast Cancer Res 8:214
Bonnefoi H, Potti A, Delorenzi M, Mauriac L, Campone M, Tubiana-Hulin M, Petit T, Rouanet P, Jassem J, Blot E, Becette V, Farmer P, Andre S, Acharya CR, Mukherjee S, Cameron D, Bergh J, Nevins JR, Iggo RD (2007) Validation of gene signatures that predict the response of breast cancer to neoadjuvant chemotherapy: a substudy of the EORTC 10994/BIG 00-01 clinical trial. Lancet Oncol 8:1071–1078
Miller WR, Larionov A, Renshaw L, Dixon JM (2009) Gene expression profiles differentiating between breast cancers clinically responsive or resistant to letrozole. J Clin Oncol 27:1382–1387
Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O’Reilly T, Stolz B, Marti A, Thomas G, Lane HA (2004) Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res 64:252–261
O’Donnell A, Faivre S, Burris HA, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588–1595
Cleator SJ, Powles TJ, Dexter T, Fulford L, Mackay A, Smith IE, Valgeirsson H, Ashworth A, Dowsett M (2006) The effect of the stromal component of breast tumours on prediction of clinical outcome using gene expression microarray analysis. Breast Cancer Res 8:R32
Murray J, Young OE, Renshaw L, White S, Williams L, Evans DB, Thomas JS, Dowsett M, Dixon JM (2009) A randomised study of the effects of letrozole and anastrozole on oestrogen receptor positive breast cancers in postmenopausal women. Breast Cancer Res Treat 114:495–501
Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, Tam L, Munro AF, Dunne B, Bartlett JM (2006) Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 48:787–794
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80
Ihaka R, Gentleman RR (1996) a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Dunning MJ, Smith ML, Ritchie ME, Tavare S (2007) Beadarray: R classes and methods for Illumina bead-based data. Bioinformatics 23:2183–2184
Johnson WE, Li C, Rabinovic A (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8:118–127
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121
Tibshirani R, Hastie T, Narasimhan B, Chu G (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA 99:6567–6572
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Sorlie T, Perou CM, Tibshirani R, AaS T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Kitchen RR, Sabine VS, Sims AH, Macaskill EJ, Renshaw L, Thomas JS, van Hemert JI, Dixon JM, Bartlett JM (2010) Correcting for intra-experiment variation in Illumina BeadChip data is necessary to generate robust gene-expression profiles. BMC Genomics 11:134
Tozlu-Kara S, Roux V, Andrieu C, Vendrell J, Vacher S, Lazar V, Spyratos F, Tubiana-Hulin M, Cohen P, Dessen P, Lidereau R, Bieche I (2007) Oligonucleotide microarray analysis of estrogen receptor alpha-positive postmenopausal breast carcinomas: identification of HRPAP20 and TIMELESS as outstanding candidate markers to predict the response to tamoxifen. J Mol Endocrinol 39:305–318
Zhao MY, Auerbach A, D’Costa AM, Rapoport AP, Burger AM, Sausville EA, Stass SA, Jiang F, Sands AM, Aguilera N, Zhao XF (2009) Phospho-p70S6K/p85S6K and cdc2/cdk1 are novel targets for diffuse large B-cell lymphoma combination therapy. Clin Cancer Res 15:1708–1720
Shah SA, Potter MW, Ricciardi R, Perugini RA, Callery MP (2001) FRAP-p70s6K signaling is required for pancreatic cancer cell proliferation. J Surg Res 97:123–130
Chuu CP, Chen RY, Barkinge JL, Ciaccio MF, Jones RB (2008) Systems-level analysis of ErbB4 signaling in breast cancer: a laboratory to clinical perspective. Mol Cancer Res 6:885–891
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790–800
Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, van de Vijver MJ, Bergh J, Piccart M, Delorenzi M (2006) Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98:262–272
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Cardoso F, van’t Veer L L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ (2008) Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol 26:729–735
Miller WR, Larionov A, Anderson TJ, Walker JR, Krause A, Evans DB, Dixon JM (2008) Predicting response and resistance to endocrine therapy: profiling patients on aromatase inhibitors. Cancer 112(3 suppl):689–694
Creighton CJ (2007) A gene transcription signature of the Akt/mTOR pathway in clinical breast tumors. Oncogene 26:4648–4655
Teschendorff AE, Naderi A, Barbosa-Morais NL, Pinder SE, Ellis IO, Aparicio S, Brenton JD, Caldas C (2006) A consensus prognostic gene expression classifier for ER positive breast cancer. Genome Biol 7:R101
Dai H, van’t Veer L, Lamb J, He YD, Mao M, Fine BM, Bernards R, van de Vijver M, Deutsch P, Sachs A, Stoughton R, Friend S (2005) A cell proliferation signature is a marker of extremely poor outcome in a subpopulation of breast cancer patients. Cancer Res 65:4059–4066
Acknowledgements
We would like to acknowledge Oliver Young, Emma Murray, Mary McHugh and Alexey Larionov for tissue banking, Edinburgh Wellcome Trust Clinical Research Facility Genetics Core for processing the BeadChips, and Edinburgh Computer and Data Facility for providing computational support. The research work was supported by Breast Cancer Research & Treatment, UK. The clinical study was funded by Novartis Pharma. AHS is funded by Breakthrough Breast Cancer.
Conflict of interest
J. Michael Dixon possesses an unrestricted educational grant and honoraria for seminar presentations from Novartis Pharma. All the other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sabine, V.S., Sims, A.H., Macaskill, E.J. et al. Gene expression profiling of response to mTOR inhibitor everolimus in pre-operatively treated post-menopausal women with oestrogen receptor-positive breast cancer. Breast Cancer Res Treat 122, 419–428 (2010). https://doi.org/10.1007/s10549-010-0928-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0928-6