Abstract
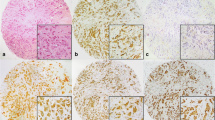
In a previous investigation reduced apoptosis was identified in normal breast tissue from cancer-containing breasts away from the cancer in comparison to age-matched normal breast from women without cancer. The hypothesis for this study was that defects in expression of apoptotic regulatory and DNA repair proteins would facilitate persistence of genetic alterations and predispose to breast cancer development. Using immunohistochemistry normal breast from 120 age-matched women (58 with breast cancer, 62 without) was analysed for proliferation, apoptosis, bcl2, BAX, caspase 3, Hsp27, Hsp70, BRCA1, ATM and BARD1. All assessments were performed without knowledge as to whether it was a cancer case or control. A significant difference was found for apoptotic index which was higher in controls (P < 0.02). There was no change in apoptotic and proliferation index with age for cancer cases unlike controls. Higher expression of bcl2 (P = 0.001) and Hsp27 (P = 0.001) was found in normal breast from cancer-containing breast in comparison to controls. There were no differences in the other proteins. Apoptosis has been found to be reduced in normal breast in a separate cohort of women with breast cancer, along with increased expression of the anti-apoptotic proteins bcl2 and Hsp27. These alterations in apoptotic regulation would enhance tumour development. Further studies are needed to examine the value of these proteins as risk markers.


Similar content being viewed by others
References
Simpson PT, Reis-Filho JS, Gale T et al (2005) Molecular evolution of breast cancer. J Pathol 205:248–254
Foote FW, Stewart FW (1945) Comparative studies of cancerous versus noncancerous breasts. Ann Surg 121:197–222
Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to precancerous lesions. J Natl Cancer Inst 55:231–273
Alpers CE, Wellings SR (1985) The prevalence of carcinoma in situ in normal and cancer associated breasts. Hum Pathol 16:796–807
Page DL, Vander Zwaag R, Rogers LW et al (1978) Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst 61:1055–1063
Dupont WD, Page DL (1985) Risk factors for breast cancer in women with proliferative disease. New Eng J Med 312:146–151
Page DL, Dupont WD (1990) Anatomic markers of human premalignancy and risk of breast cancer. Cancer 66:1326–1335
Tavassoli FA, Norris AJ (1990) A comparison of the results of long-term follow-up for atypical ductal hyperplasia and intraduct hyperplasia of the breast. Cancer 65:518–529
McDivitt RW, Stevens JA, Lee NC et al (1992) Histologic types of benign breast disease and the risk of breast cancer. Lancet 69:1408–1414
London SJ, Connolly JL, Schnitt SJ et al (1992) A prospective study of benign breast disease and the risk of breast cancer. J Am Med Assoc 267:941–944
Wang J, Constantino JP, Tan-Chin E et al (2004) Lower category benign breast disease and the risk of invasive breast cancer. J Natl Cancer Inst 96:616–620
Slaughter DP, Southwick HW, Smejkal W (1953) Field cancerisation in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 6:963–968
Braakhuis BJM, Tabor MP, Kummer JA et al (2003) A genetic explanation of Slaughter’s concept of field cancerisation: evidence and clinical implications. Cancer Res 63:1727–1730
Deng G, Lu Y, Zlotnikov G et al (1996) Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 274:2057–2059
Larson PS, de las Morenas A, Cupples LA (1998) Genetically abnormal clones in histologically normal breast tissue. Am J Pathol 152:1591–1598
Lakhani SR, Chaggar R, Davies S et al (1999) Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J Pathol 189:496–503
Larson PS, de las Morenas A, Bennett SR et al (2002) Loss of heterozygosity or allele imbalance in histologically normal breast epithelium is distinct from loss of heterozygosity or allele imbalance in co-existing carcinomas. Am J Pathol 161:283–290
Tlsty TD, Crawford YG, Holst CR et al (2004) Genetic and epigenetic changes in mammary epithelial cells may mimic early events in carcinogenesis. J Mammary Gland Biol Neoplasia 9:263–274
Ellsworth DL, Ellsworth RE, Liebman MN et al (2004) Genomic instability in histologically normal breast tissues: implications for carcinogenesis. Lancet Oncol 5:753–758
Larson PS, Schlecter BL, de las Morenas et al (2005) Allele imbalance, or loss of heterozygosity, in normal breast epithelium of sporadic breast cancer cases and BRCA1 gene mutation carriers is increased compared with reduction mammoplasty tissues. J Clin Oncol 23:8613–8619
Walker RA, Cowl J, Dhadly PPS et al (1992) Oestrogen receptor, epidermal growth factor receptor and oncoprotein expression in non-involved tissue of cancerous breasts. Breast 2:87–91
Jones JL, Critchley DR, Walker RA (1992) Alteration of integrin and stromal protein expression—a marker of pre-malignant change? J Pathol 167:399–406
Hassan HI, Walker RA (2001) Altered expression of epidermal growth factor receptor in non-involved tissue of cancer-containing breasts. Breast 10:318–324
Hassan HI, Walker RA (1998) Decreased apoptosis in non-involved tissue from cancer-containing breasts. J Pathology 184:258–264
Allan DJ, Howell A, Roberts SA et al (1992) Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human breast. J Pathol 167:25–32
Clarke RB, Howell A, Potten CS et al (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57:4987–4991
Shoker BS, Jarvis C, Clarke RB et al (1999) Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 155:1811–1815
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337
Schorr K, Li M, Bar-Peled U et al (1999) Gain of Bcl-2 is more potent than BAX loss in regulating mammary epithelial cell survival in vivo. Cancer Res 59:2541–2545
Sabourin JC, Martin A, Baruch J et al (1994) Bcl-2 expression in normal breast during the menstrual cycle. Int J Cancer 59:1–6
Gompel A, Somai S, Chaout M et al (2000) Hormonal regulation of apoptosis in breast cells and tissues. Steroids 65:593–598
Gee JM, Robertson JFR, Ellis IO et al (1994) Immunocytochemical localization of bcl-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer 59:619–628
Ciocca DR, Oesterreich S, Chamness GC et al (1993) Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst 85:1558–1570
Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8:61–70
Konishi H, Matsuzaki H, Tamaka M et al (1997) Activation of protein kinase B (AKT/RAC-protein kinase) by cellular stress and its association with heat shock protein HSP27. FEBS Lett 410:493–498
O’Neill PA, Shaaban AM, West CR et al (2004) Increased risk of malignant progression in benign proliferating breast lesions defined by expression of heat shock protein 27. Br J Cancer 90:182–188
Taylor J, Lymboura M, Pace PE et al (1998) An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. Int J Cancer 79:334–342
Lambie H, Miremadi A, Pinder SE et al (2003) Prognostic significance of BRCA1 expression in sporadic breast carcinomas. J Pathol 200:207–213
Angele S, Treilleux I, Taniere P et al (2000) Abnormal expression of the ATM and P53 genes in sporadic breast carcinomas. Clin Cancer Res 6:3536–3544
Angele S, Treilleux I, Bremond A et al (2003) Altered expression of DNA double-strand break detection and repair proteins in breast carcinomas. Histopathology 43:347–353
Angele S, Jones C, Reis Filho JS et al (2004) Expression of ATM, p53 and the MRE11-Rad50-NBSI complex in myoepithelial cells from benign and malignant proliferations of the breast. J Clin Pathol 57:1179–1184
Clarke RA, Kairouz R, Watters D et al (1998) Upregulation ATM in sclerosing adenosis of the breast. Mol Pathol 51:224–226
Yan PS, Venkataramu C, Ibrahim A et al (2006) Mapping geographic zones of cancer risk with epigenetic biomarkers in normal breast tissue. Clin Cancer Res 12:6626–6636
Fabian CJ, Kimler BF, Mayo MS et al (2005) Breast-tissue sampling for risk assessment and prevention. Endocr Res Cancer 12:185–213
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batchelder, A.J., Gordon-Weeks, A.N. & Walker, R.A. Altered expression of anti-apoptotic proteins in non-involved tissue from cancer-containing breasts. Breast Cancer Res Treat 114, 63–69 (2009). https://doi.org/10.1007/s10549-008-9988-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-9988-2