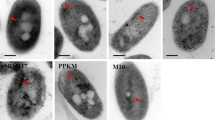
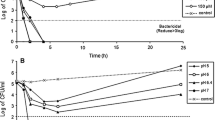
Abstract
Streptococcus pneumoniae (pneumococcus) is responsible for nearly one million child deaths annually. Pneumococcus causes infections such as pneumonia, otitis media, meningitis, and sepsis. The human immune system includes antibacterial peptides and proteins such as lactoferrin (LF), but its activity against pneumococcus is not fully understood. The aim of this work was to evaluate the bactericidal effect of bovine lactoferrin (bLF) and the synthetic LF-peptides lactoferricin (LFcin17–30), lactoferrampin (LFampin265–284), and LFchimera against S. pneumoniae planktonic cells. The mechanism of damage was also investigated, as well as the impact of these peptides on the transcription levels of genes known to encode important virulence factors. S. pneumoniae planktonic cells were treated with bLF, LFcin17–30, LFampin265–284 and LFchimera at different time points. The viability of treated planktonic cells was assessed by dilution and plating (in CFU/ml). The interaction between LF and LF-peptides coupled to fluorescein was visualized using a confocal microscope and flow cytometry, whereas the damage at structural levels was observed by electron microscopy. Damage to bacterial membranes was further evaluated by membrane permeabilization by use of propidium iodide and flow cytometry, and finally, the expression of pneumococcal genes was evaluated by qRT-PCR. bLF and LFchimera were the best bactericidal agents. bLF and peptides interacted with bacteria causing changes in the shape and size of the cell and membrane permeabilization. Moreover, the luxS gene was down-regulated in bacteria treated with LF. In conclusion, LF and LFchimera have a bactericidal effect, and LF down-regulates genes involved in the pathogenicity of pneumococcus, thus demonstrating potential as new agents for the treatment of pneumococcal infections.





Similar content being viewed by others
References
Abraham WR (2006) Controlling biofilms of Gram-positive pathogenic bacteria. Curr Med Chem 13(13):1509–1524
Ammons MC, Copie V (2013) Mini-review: lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling 29(4):443–455. doi:10.1080/08927014.2013.773317
Arnold RR, Brewer M, Gauthier JJ (1980) Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun 28(3):893–898
Avery OT, Macleod CM, McCarty M (1944) Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med 79(2):137–158
Bolscher JG, Adao R, Nazmi K, van den Keybus PA, van’t Hof W, Amerongen AVN, Bastos M, Veerman EC (2009) Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 91(1):123–132. doi:10.1016/j.biochi.2008.05.019
Bolscher J, Nazmi K, van Marle J, van’t Hof W, Veerman E (2012) Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem Cell Biol 90(3):378–388. doi:10.1139/o11-085
Cavestro GM, Ingegnoli AV, Aragona G, Iori V, Mantovani N, Altavilla N, Dal Bo N, Pilotto A, Bertele A, Franze A, Di Mario F, Borghi L (2002) Lactoferrin: mechanism of action, clinical significance and therapeutic relevance. Acta Biomed 73(5–6):71–73
Cvitkovitch DG, Li YH, Ellen RP (2003) Quorum sensing and biofilm formation in streptococcal infections. J Clin Invest 112(11):1626–1632. doi:10.1172/JCI20430
Domenech M, Ramos-Sevillano E, Garcia E, Moscoso M, Yuste J (2013) Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae. Infect Immun 81(7):2606–2615. doi:10.1128/IAI.00491-13
Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltran J, Leon-Sicairos N, Bolscher JG (2010) Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23(3):569–578. doi:10.1007/s10534-010-9306-4
Flores-Villasenor H, Canizalez-Roman A, de la Garza M, Nazmi K, Bolscher JG, Leon-Sicairos N (2012a) Lactoferrin and lactoferrin chimera inhibit damage caused by enteropathogenic Escherichia coli in HEp-2 cells. Biochimie 94(9):1935–1942. doi:10.1016/j.biochi.2012.05.011
Flores-Villasenor H, Canizalez-Roman A, Velazquez-Roman J, Nazmi K, Bolscher JG, Leon-Sicairos N (2012b) Protective effects of lactoferrin chimera and bovine lactoferrin in a mouse model of enterohaemorrhagic Escherichia coli O157:H7 infection. Biochem Cell Biol 90(3):405–411. doi:10.1139/o11-089
Haukland HH, Ulvatne H, Sandvik K, Vorland LH (2001) The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett 508(3):389–393
Ho YH, Sung TC, Chen CS (2011) Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol Cell Proteomics 11(4):M111-014720. doi:10.1074/mcp.M111.014720
Huo L, Zhang K, Ling J, Peng Z, Huang X, Liu H, Gu L (2011) Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch Oral Biol 56(9):869–876. doi:10.1016/j.archoralbio.2011.02.004
Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME (2007) Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 189(1):38–51
Leon-Sicairos N, Canizalez-Roman A, de la Garza M, Reyes-Lopez M, Zazueta-Beltran J, Nazmi K, Gomez-Gil B, Bolscher JG (2009) Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91(1):133–140. doi:10.1016/j.biochi.2008.06.009
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Lopez-Soto F, Leon-Sicairos N, Nazmi K, Bolscher JG, de la Garza M (2010) Microbicidal effect of the lactoferrin peptides lactoferricin17–30, lactoferrampin265–284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals 23(3):563–568. doi:10.1007/s10534-010-9295-3
Lynch JP 3rd, Zhanel GG (2010) Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med 16(3):217–225. doi:10.1097/MCP.0b013e3283385653
Mirza S, Wilson L, Benjamin WH Jr, Novak J, Barnes S, Hollingshead SK, Briles DE (2011) Serine protease PrtA from Streptococcus pneumoniae plays a role in the killing of S. pneumoniae by apolactoferrin. Infect Immun 79(6):2440–2450. doi:10.1128/IAI.00489-10
Mookherjee N, Hancock RE (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64(7–8):922–933. doi:10.1007/s00018-007-6475-6
Moscoso M, Garcia E, Lopez R (2009) Pneumococcal biofilms. Int Microbiol 12(2):77–85
Nijnik A, Hancock R (2009) Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg Health Threats J 2:e1. doi:10.3134/ehtj.09.001
Nunes MC, Shiri T, van Niekerk N, Cutland CL, Groome MJ, Koen A, von Gottberg A, de Gouveia L, Klugman KP, Adrian PV, Madhi SA (2013) Acquisition of Streptococcus pneumoniae in pneumococcal conjugate vaccine-naive South African children and their mothers. Pediatr Infect Dis J. doi:10.1097/INF.0b013e31828683a3
Orsi N (2004) The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17(3):189–196
Shak JR, Ludewick HP, Howery KE, Sakai F, Yi H, Harvey RM, Paton JC, Klugman KP, Vidal JE (2013) Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. MBio 4(5):e00655-13. doi:10.1128/mBio.00655-13
Shaper M, Hollingshead SK, Benjamin WH Jr, Briles DE (2004) PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected]. Infect Immun 72(9):5031–5040. doi:10.1128/IAI.72.9.5031-5040.2004
Silva T, Abengozar MA, Fernandez-Reyes M, Andreu D, Nazmi K, Bolscher JG, Bastos M, Rivas L (2012) Enhanced leishmanicidal activity of cryptopeptide chimeras from the active N1 domain of bovine lactoferrin. Amino Acids 43(6):2265–2277. doi:10.1007/s00726-012-1304-0
Silva T, Adao R, Nazmi K, Bolscher JG, Funari SS, Uhrikova D, Bastos M (2013) Structural diversity and mode of action on lipid membranes of three lactoferrin candidacidal peptides. Biochim Biophys Acta 1828(5):1329–1339. doi:10.1016/j.bbamem.2013.01.022
Talekar SJ, Chochua S, Nelson K, Klugman KP, Quave CL, Vidal JE (2014) 220D-F2 from Rubus ulmifolius kills Streptococcus pneumoniae planktonic cells and pneumococcal biofilms. PLoS One 9(5):e97314. doi:10.1371/journal.pone.0097314
Tomita M, Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K (1991) Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci 74(12):4137–4142. doi:10.3168/jds.S0022-0302(91)78608-6
Tomita M, Takase M, Wakabayashi H, Bellamy W (1994) Antimicrobial peptides of lactoferrin. Adv Exp Med Biol 357:209–218
Ulvatne H, Samuelsen O, Haukland HH, Kramer M, Vorland LH (2004) Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol Lett 237(2):377–384. doi:10.1016/j.femsle.2004.07.001
Valenti P, Berlutti F, Conte MP, Longhi C, Seganti L (2004) Lactoferrin functions: current status and perspectives. J Clin Gastroenterol 38(6 Suppl):S127–S129
van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25(2):177–183. doi:10.1016/j.peptides.2003.12.006
Vidal JE, Ohtani K, Shimizu T, McClane BA (2009) Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell Microbiol 11(9):1306–1328. doi:10.1111/j.1462-5822.2009.01332.x
Vidal JE, Ludewick HP, Kunkel RM, Zahner D, Klugman KP (2011) The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect Immun 79(10):4050–4060. doi:10.1128/IAI.05186-11
Vidal JE, Howery KE, Ludewick HP, Nava P, Klugman KP (2013) Quorum-sensing systems LuxS/autoinducer 2 and Com regulate Streptococcus pneumoniae biofilms in a bioreactor with living cultures of human respiratory cells. Infect Immun 81(4):1341–1353. doi:10.1128/IAI.01096-12
Vogel HJ (2012) Lactoferrin, a bird’s eye view. Biochem Cell Biol 90(3):233–244. doi:10.1139/o2012-016
Acknowledgments
We thank to BS Lourdes Rojas-Morales and BS Sirenia González-Pozos for their technical assistance in the Microscopy Unit of CINVESTAV-IPN, México. Authors also thank Gideon Matzkin, Emory University, for his valuable support in some laboratory procedures. This work was supported by grants from CONACYT (CB-2009-133677) and PROFAPI-UAS (2012/087; 2013/093).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
León-Sicairos, N., Angulo-Zamudio, U.A., Vidal, J.E. et al. Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. Biometals 27, 969–980 (2014). https://doi.org/10.1007/s10534-014-9775-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9775-y