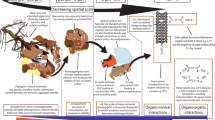
Abstract
Rock colonization by plant roots and their associated microbiota is one of the major drivers of mineral weathering, nutrient cycling, soil formation and ecosystem stability. Yet the mechanisms of bio-uptake of lithogenic elements from rocks with differential nutrient availabilities and limitations are yet to be established. Here we present results from a mesocosm experiment that examined lithogenic element dissolution and uptake (P, K, Ca, Mg, Mn, Fe, Na, Ti, Al and Si) in Bouteloua dactyloides (buffalo grass) grown on four different granular porous media (basalt, rhyolite, granite and schist) comprised of primary mineral assemblages as influenced by arbuscular mycorrhiza (AM; Rhizophagus irregularis). Our results demonstrated that nutrient mobilization (chemical denudation + plant uptake) in such oligotrophic systems is governed by nutrient supply in the parent material, nutrient availability in pore water solution, and plant physiology. Overall, total major lithogenic element mobilization in planted columns (with and without AM) exceeded abiotic controls in all substrates. Differences in total mobilization among substrates occurred as follows: Fe, Na, Ti and Al reached high values in planted treatments in basalt, P and Mn in rhyolite, Ca and K in granite and K in schist, suggesting enhanced dissolution of primary minerals in the presence of plants. Element biomass enrichment of Mn, Fe, Ti and Al appeared to be higher in basalt than the rest of the substrates; however, high Al availability limited Ca and Mg uptake and plant growth in this rock media. Presence of mycorrhiza enhanced shoot biomass in rhyolite due to increased P uptake, and increased concentrations and total uptake of lithogenic elements in plants in all rocks but granite. As expected, AM significantly increased plant root concentrations of P, K, Ca, Mn, Fe, Ti, Al in basalt, and Mn shoot concentrations in rhyolite, as well as root total uptake of K, Ca, Mg, Mn, Fe, Na, Ti, Al and Si in basalt. At the same time, AM decreased Ca, Ti and Al concentrations in shoots grown in rhyolite, a possible protection mechanism against Al toxicity. The importance of AM in nutrient uptake is also reinforced by positive correlations between AM infection rate and P, Ca and Mn total uptake across all substrates. Moreover, total mobilization of Ca, Mg and Mn in rhyolite, was significantly higher in the AM versus non-AM treatment, contrary to K, Ca, Mg, Na and Si in schist. Our work demonstrates how mineral weathering and associated nutrient release is promoted by plant processes, further enhanced by plant associated with symbiotic AM, and yet more pronounced in basalt and rhyolite compared to granite and schist.







Similar content being viewed by others
References
Akter M, Akagi T (2005) Effect of fine root contact on plant-induced weathering of basalt. J Soil Sci Plant Nutr 51:861–871
Akter M, Akagi T (2010) Dependence of plant-induced weathering of basalt and andesite on nutrient conditions. Geochem J 44:137–150
Amundson R, Richter DD, Humphreys GS, Jobbágy EG, Gaillardet J (2007) Coupling between biota and earth materials in the critical zone. Elements 3:327–332
Anderson DW (1988) The effect of parent material and soil development on nutrient cycling in temperate ecosystems. Biogeochemistry 5:51–97
Arocena JM, Velde B, Robertson SJ (2012) Weathering of biotite in the presence of arbuscular mycorrhizae in selected agricultural crops. Appl Clay Sci 64:12–17
Azcón R, Rubio R, Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol 117:399–404
Balogh-Brunstad Z, Keller CK, Dickinson JT, Stevens F, Li CY, Bormann BT (2008) Biotite weathering and nutrient uptake by ectomycorrhizal fungus, Suillus tomentosus, in liquid-culture experiments. Geochim Cosmochim Acta 72:2601–2618
Bashan Y, Vierheilig H, Salazar BG, de-Bashan LE (2006) Primary colonization and breakdown of igneous rocks by endemic, succulent elephant trees (Pachycormus discolor) of the deserts in Baja California, Mexico. Naturwissenschaften 93:344–347
Bashan Y, Khaosaad T, Salazar BG, Ocampo JA, Wiemken A, Oehl F, Vierheilig H (2007) Mycorrhizal characterization of the boojum tree, Fouquieria columnaris, an endemic ancient tree from the Baja California Peninsula, Mexico. Trees-StructFunct 21:329–335
Bonneville S, Morgan DJ, Schmalenberger A, Bray A, Brown A, Banwart SA, Bening LG (2011) Tree-mycorrhiza symbiosis accelerate mineral weathering: evidences from nanometer-scale elemental fluxes at the hypha-mineral interface. Geochim Cosmochim Acta 75:6988–7005
Brantley SL, Megonigal JP, Scatena FN, Balogh-Brunstad Z, Barnes RT, Bruns MA, Van Cappellen P, Dontsova K, Hartnett HE, Hartshorn AS, Heimsath A, Herndon E, Jin L, Keller CK, Leake JR, McDowell WH, Meinzer FC, Mozdzer TJ, Petsch S, Pett-Ridge J, Pregitzer KS, Raymond PA, Riebe CS, Shumaker K, Sutton-Grier A, Walter R, Yoo K (2011) Twelve testable hypotheses on the geobiology of weathering. Geobiology 9:140–165
Calvaruso C, Turpault M-P, Frey-Klett P, Uroz S, Pierret M-C, Tosheva Z, Kies A (2013) Increase of apatite dissolution rate by Scots pine roots associated or not with Burkholderiaglathei PML1(12)Rp in open-system flow microcosms. Geochim Cosmochim Acta 106:287–306
Caris C, Hördt W, Hawkins HJ, Römheld V, George E (1998) Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza 8:35–39
Chorover J, Kretzschmar R, Garcia-Pichel F, Sparks DL (2007) Soil biogeochemical processes within the critical zone. Elements 3:321–326
Cumming JR, Ning J (2003) Arbuscular mycorrhizal fungi enhance aluminium resistance of broom sedge (Andropogon virginicus L.). J Exp Bot 54:1447–1459
Dontsova K, Zaharescu D, Henderson W, Verghese S, Perdrial N, Hunt E, Chorover J (2014) Impact of organic carbon on weathering and chemical denudation of granular basalt. Geochim Cosmochim Acta. doi:10.1016/j.gca.2014.05.010
Eckardt NA (2005) Insights into plant cellular mechanisms: of phosphatase transporters and arbuscular mycorrhizal infection. Plant Cell 17:3213–3216
El-Nashaar HM, Griffith SM, Steiner JJ, Banowetz GM (2009) Mineral concentration in selected native temperate grasses with potential use as biofuel feedstock. Bioresour Technol 100:3526–3531
Eskandari A, Danesh YR (2010) Study on life cycle of arbuscular mycorrhizal fungus Glomus intraradices using in vitro culturing technique. JPhytol 2:69–75
Göransson A (2001) A technique for quantitative trace element and micronutrient studies in plants. In: Gobran GR, Wenzel WW, Lombi E (eds) Trace elements in the rhizosphere (2001). CRC Press LLC, Florida, pp 220–221
Hall K, Arocena JM, Boelhouwers J, Liping Z (2005) The influence of aspect on the biological weathering of granites: observations from the Kunlun Mountains, China. Geomorphology 67:171–188
Harley AD, Gilkes RJ (2000) Factors influencing the release of plant nutrient elements from silicate rock powders: a geochemical overview. Nutr Cycl Agroecosyst 56:11–36
Haselwandter K (1995) Mycorrhizal fungi: siderophore production. Crit Rev Biotechnol 15:287–291
Hinsinger P, Plassard C, Tang C, Jailard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol 168:293–303
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Hoffland E, Giesler R, Jongmans AG, Van Breemen N (2003) Feldspar tunneling by fungi along natural productivity gradients. Ecosystems 6:739–746
Kabata-Pendias A, Pendias H (2000) Trace elements in soils and plants, 2ndedn edn. CRC Press, Florida
Klugh KR, Cumming JR (2007) Variations in organic acid exudation and aluminum resistance among arbuscular mycorrhizal species colonizing Liriodendron tulipifera. Tree Physiol 27:1103–1112
Koele N, Dickie IA, Blum JD, Gleason JD, De Graaf L (2014) Ecological significance of mineral weathering in ectomycorrhizal and arbuscular mycorrhizal ecosystems from a field-based comparison. Soil Biol Biochem 69:63–70
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lehmann A, Rillig MC (2015) Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—a meta-analysis. Soil Biol Biochem 81:147–158
Lindberg S, Strid H (1997) Aluminium induces rapid changes in cytosolic pH and free calcium and potassium concentrations in root protoplasts of wheat. Physiol Plant 99:405–414
Lopez BR, Bashan Y, Bacilio M (2009) Rock colonization plants: abundance of the endemic cactus Mammillaria fraileana related to rock type in the southern Sonoran desert. Plant Ecol 201:575–588
Lux HB, Cummings JR (2001) Mycorrhizae confer aluminum resistance to tulip-poplar seedlings. Can J Forest Res 31:694–702
Mapelli F, Marasco R, Balloi A, Rolli E, Cappitelli F, DaffonchioD Borin S (2012) Mineral–microbe interactions: biotechnological potential of bioweathering. J Biotechnol 157:473–481
Marschner H (2002) Mineral nutrition of higher plants, 2nd edn edn. Academic Press, London
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2009) Effects of arbuscular mycorrhiza, soil sterilization, and soil compaction on wheat (Triticumaestivum L.) nutrient uptake. Soil Tillage Res 104:48–55
Moffet CA (2003) Competition among blue grama and buffalo grass ecotypes: effects of soil and past neighbor interactions. PhD Thesis, Texas Tech University
Mora ML, Alfaro MA, Jarvis SC, Demanet R, Cartes P (2006) Soil aluminum availability in andisols of Southern Chile and its effect on forage production and animal metabolism. Soil Use Manag 22:95–101
Nichol BE, Oliveira LA, Glass ADM, Siddiqi MY (1993) The effects of aluminum on the influx of calcium, potassium, ammonium, nitrate, and phosphate in an aluminium-sensitive cultivar of barley (Hordeum vulgare). Plant Physiol 101:1263–1266
Olsson-Francis K, Simpson AE, Wolff-Boenisch D, Cockell CS (2012) The effect of rock composition on cyanobacterial weathering of crystalline basalt and rhyolite. Geobiology 10:434–444
Perdrial N, Rivera N, Thompson A, O’Day PA, Chorover J (2011) Trace contaminant concentration affects mineral transformation and pollutant fate in hydroxide-weathered Hanford sediments. J Hazard Mater 197:119–127
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:157–160
Puente ME, Bashan Y, Li CY, Lebsky VK (2004a) Microbial populations and activities in the rhizoplane of rock-weathering desert plants, I. Root colonization and weathering of igneous rocks. Plant Biol 6:629–642
Puente ME, Li CY, Bashan Y (2004b) Microbial populations and activities in the rhizoplane of rock-weathering desert plants, II. Growth promotion of cactus seedling. Plant Biol 6:643–650
Quirk J, Beerling DJ, Banwart SA, Kakonyi G, Romero-Gonzalez ME, Leake JR (2012) Evolution of trees and mycorrhizal fungi intensifies silicate mineral weathering. Biol Lett 8:1006–1011
Rosling A, Lindahl BD, Taylor AFS, Finlay RD (2004) Mycelial growth and substrate acidification of ectomycorrhizal fungi in response to different minerals. FEMS Microbiol Ecol 47:31–37
Solis-Dominguez FA, White SA, Hutter TB, Amistadi MK, Root RA, Chorover J, Maier R (2012) Response of key soil parameters during compost-assisted phyto stabilization in extremely acidic tailings: effect of plant species. Environ Sci Technol 46:1019–1027
Song W, Ogawa N, Takashima-Oguchi C, Hatta T, Matsukura Y (2010) Laboratory experiments on bacterial weathering of granite and its constituent minerals. Géomorphologie 4:327–336
Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ (2009) Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7:171–191
Thompson K, Parkinson JA, Band SR, Spencer RE (1997) A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol 136:679–689
Turnau K, Kottke I, Oberwinkler F (1993) Elemental localization in mycorrhizal roots of Pteridium aquilinium (L.) Kuhn. collected from experimental plots treated with Cd dust. New Phytol 123:313–324
Verboom WH, Pate JS (2013) Exploring the biological dimension to pedogenesis with emphasis on the ecosystems, soils and landscapes of southwestern Australia. Geoderma 211–212:154–183
Wang MY, Xia RX (2009) Effects of arbuscular mycorrhizal fungi on growth and iron uptake of Poncirus trifoliata under different pH. Acta Microbiol Sin 49:1374–1379
Weaver JE (1954) North American Prairie. Johnsen Publishing Company, Lincoln
Whitehead DC (2000) Nutrient elements in grassland, soil-plant-animal relationships. CABI Publishing, Wallingford
Wilson MJ (2004) Weathering of the primary rock-forming minerals: processes, products and rates. Clay Miner 39:233–266
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Porter H, Villar R, Warton DI, Westoby M (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wu QS, Li GH, Zou YN (2011) Roles of arbuscular mycorrhizal fungi on growth and nutrient acquisition of peach (Prunus Persica L. Batsch) seedlings. J Anim Plant Sci 21:746–750
Acknowledgments
This research was supported by National Science Foundation Grants no. EAR-1023215 and EAR-1331408 (which supports the Catalina-Jemez CZO), Philecology Foundation of Fort Worth Texas, and University of Arizona Brown Foundation. Part of this research was carried out at the Stanford Synchrotron Radiation Laboratory (SSRL), a National User Facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. We are very grateful to Mary Kay Amistadi for performing ICP-MS analysis on plant digests, Edward Hunt for helping with water chemistry analysis, Nicolas Perdrial for the help with XRD analysis, Kenneth Domanik for the help with microprobe analysis, Kolja Schuh for his suggestions on statistical analyses, Julia Perdrial and two anonymous reviewers for the useful comments and suggestions on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Sharon A
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burghelea, C., Zaharescu, D.G., Dontsova, K. et al. Mineral nutrient mobilization by plants from rock: influence of rock type and arbuscular mycorrhiza. Biogeochemistry 124, 187–203 (2015). https://doi.org/10.1007/s10533-015-0092-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0092-5