Abstract
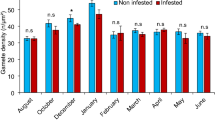
The intertidal mussel Mytilus galloprovincialis is a successful invader worldwide. Since its accidental introduction onto the South African west coast in the late 1970s, it has become the most successful marine invasive species in South Africa. One possible explanation for this phenomenon is that M. galloprovincialis suffers less from phototrophic shell-degrading endoliths in its invasive than in its native range. We assessed photoautotrophic endolithic pressure on M. galloprovincialis in native (Portugal) and invasive (South Africa) ranges. Invasive populations were more heavily infested than native populations. In Portugal, only the biggest/oldest mussels displayed endolithic erosion of the shell and the incidence of infestation was greater at higher shore levels where more prolonged exposure to light enhances endolith photosynthesis. In South Africa, even the smallest size classes of mussels were heavily infested throughout the shore. In Portugal, endolithic-induced mortality was observed at only one location, while in South Africa it occurred at all locations and at significantly higher rates than in Portugal. Important sub-lethal effects were detected in infested native mussels, confirming previous studies of invasive populations and suggesting an energy trade-off between shell repair and other physiological constraints. We observed a positive relationship between infestation rates and barnacle colonization on mussel shells, suggesting possible facilitation of barnacle settlement/survival by shell-boring pathogens. Identification of endoliths revealed common species between regions. However, two species were unique in the invasive range while another was unique in the native region. Different levels of endolithic infestation in the invasive and the native range were not explained by the effect of major environmental determinants (Photosynthetically Available Radiation and wave height). The results reject our initial hypothesis, indicating that invasion success of M. galloprovincialis is not simply explained by escape from its natural enemies but results from complex interactions between characteristics of the invaded community and properties of the invader.









Similar content being viewed by others
References
Abada-Boudjema Y, Dauvin J (1995) Recruitment and life span of two natural mussel populations Perna perna (Linnaeus) and Mytilus galloprovincialis (Lamarck) from the Algerian coast. J Mollus Stud 61:467–481
Alfaro AC, Webb SC, Barnaby C (2008) Variability of growth, health, and population turnover within mussel beds of Perna canaliculus in northern New Zealand. Mar Biol Res 4:376–383
Ambariyanto A, Seed R (1991) The infestation of Mytilus edulis Linneaus by Polydora ciliata (Johnston) in the Conwy estuary, North Wales. J Mollus Stud 57:413–424
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Astenei I, Gosling E, Wilson J, Powell E (2005) Genetic variability and phylogeography of the invasive zebra mussel, Dreissena polymorpha (Pallas). Mol Ecol 14:1655–1666
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260
Bers AV, Diaz ER, de Gama BAP, Vieira-Silva F, Dobretsov S, Valdivia N, Thiel M, Scardino AJ, McQuaid CD, Sudgen HE, Thomason JC, Wahl M (2010) Relevance of mytilid shell microtopographies for fouling defence—a global comparison. Biofouling 26:367–377
Boaventura D, Ré P, Cancela da Fonseca L, Hawkins SJ (2002) Intertidal rocky shore communities of the continental Portuguese coast: analysis of distribution patterns. Mar Ecol 23(1):69–90
Bownes SJ, McQuaid CD (2006) Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. the south coast of South Africa? J Exp Mar Biol Ecol 338:140–151
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215
Calvo-Ugarteburu G, McQuaid CD (1998a) Parasitism and invasive species: effects of digenetic trematodes on mussels. Mar Ecol Prog Ser 169:149–163
Calvo-Ugarteburu G, McQuaid CD (1998b) Parasitism and introduced species: epidemiology of trematodes in the intertidal mussels Perna perna and Mytilus galloprovincialis. J Exp Mar Biol Ecol 220:47–65
Carr DE, Eubanks MD (2002) Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae). Evolution 56:22–30
Carvalho D, Oliveira JT, Pereira E, Ramalho M, Antunes MT, Monteiro JH (1992) Carta Geológica de Portugal na escala 1:500.000. Folha Sul Serv Geol de Portugal, Lisboa
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis. Ecol Lett 7:721–733
Costa M (1995) Agitação marítima na costa portuguesa. An Inst Hidrogr 13:35–40
Cristescu MEA, Witt JDS, Grigorovish IA, Hebert PDN, MacIssac HJ (2004) Dispersal of the Ponto-Caspian amphipod Echinogammarus ischnus: invasion waves from the Pleistocene to the present. Heredity 92:197–203
Da Silva CM, Cachão M, Martinell J, Domènech R (1999) Bioerosional evidence of rocky polaeoshores in the Neogene of Portugal: environmental and stratigraphical significance. Bull Geol Soc Den 45:156–160
Davenport J, Chen X (1987) A companson methods for the assessment of condition in the mussel (Mytilus edulis L.). J Mollus Stud 53:293–297
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Denny MW (1987) Lift as a mechanism of patch initiation in mussel beds. J Exp Mar Biol Ecol 113:231–245
DeWalt SJ, Denslow JS, Ickes K (2004) Natural enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology 85:471–483
Friedmann EI, Ocampo-Friedmann R (1984) Endolithic microorganisms in extreme dry environments: analysis of lithobiontic microbial habitat pp 177–185. In: Klug MJ, Reddy CA (ed) Current perspectives in microbial ecology. Am Soc Microbiol, Washington
Gektidis M, Dubinsky Z, Goffredo S (2007) Microendoliths ofthe shallow euphotic zone inopenandshadedhabitatsat30 N -Eilat, Israel- paleoecological implications. Facies 53:43–55
Geller JB (1990) Reproductive responses to shell damage by the gastropod Nucella emarginata. J Exp Mar Biol Ecol 136:77–87
Geller JB (1999) Decline of a native mussel masked by sibling species invasion. Conserv Biol 13:661–664
Golani DG, Azzueeo E, Corsini-Foka M, Falautano M, Andaloro F, Bernardi G (2007) Genetic bottlenecks and successful biological invasions: the case of a recent Lessepsian migrant. Biol Lett 3:541–545
Golubic S, Friendmann I, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. Sediment Geol 51:475–478
Golubic S, Radtke G, Le Campion-Alsumard T (2005) Endolithic fungi in marine ecosystems. Trends Microbiol 13:229–235
Grant WS, Cherry MI (1985) Mytilus galloprovincialis Lmk. in Southern Africa. J Exp Biol 90:179–191
Griffiths CL, Hockey PAR, van Erkom Schurink C, Le Roux PJ (1992) Marine invasive aliens on South African rocky shores: implications for community structure and trophic functioning. S Afr J Mar Sci 12:713–722
Hanekom N (2008) Invasion of an indigenous Perna perna mussel bed on the south coast of South Africa by an alien mussel Mytilus galloprovincialis ans its effect on the associated fauna. Biol Invasions 10:233–244
Harris JM, Branch GM, Elliot BL, Currie B, Dye AH, McQuaid CD, Tomalin BJ, Velasquez C (1998) Spatial and temporal variability in recruitment of intertidal mussels around the coast of southern Africa. S Afr J Zool 33:1–11
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR (1999) Emerging marine diseases-climate links and anthropogenic factors. Science 285:1505–1510
Hatcher MJ, Dunn AM (2011) Parasites in ecological communities: from interactions to ecosystems. Cambridge University Press, Cambridge
Hatcher MJ, Dick JTA, Dunn AM (2006) How parasites affect interactions between competitors and predators. Ecol Lett 9:1253–1271
Heger T, Trepl L (2003) Predicting biological invasions. Biol Invasions 5:313–321
Hilbish TJ, Mullinax A, Dolven SI, Meyer A, Koehn RK, Rawson PD (2000) Origin of the antitropical distribution pattern in marine mussels (Mytilus spp.): routes and timing of transequatorial migration. Mar Biol 136:69–77
Hockey PAR, van Erkom Schurink C (1992) The invasive biology of the mussel Mytilus galloprovincialis on the southern African coast. Trans R Soc S Afr 48:123–139
Hudson PJ, Greenman JV (1998) Competition mediated by parasites: biological and theoretical progress. Trends Ecol Evol 13:387–390
Ishtiaq F, Beadell JS, Baker AJ, Rahmani AR, Jhala YV, Fleischer RC (2006) Prevalence and evolutionary relationships of haematozoan parasites in native versus introduced populations of common myna Acridotheres tristis. Proc R Soc Lond B 273:587–594
Kaehler S (1999) Incidence and distribution of phototrophic shell-degrading endoliths of the brown mussel Perna Perna. Mar Biol 135:505–514
Kaehler S, McQuaid CD (1999) Lethal and sub-lethal effects of phototrophic endoliths attacking the shell of the intertidal mussel Perna perna. Mar Biol 135:497–503
Kelly DW, Muirhead JR, Heath DD, Macisaac HJ (2006) Contrasting patterns in genetic diversity following multiple invasions of fresh and brackish waters. Mol Ecol 15:3641–3653
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 19:199–204
Lafferty KD, Kuris AM (1996) Biological control of marine pests. Ecology 77:1989–2000
Laukner G (1983) Diseases of Mollusca: Bivalvia. In: Kinne O (ed) Diseases of marine animals. Biologische Anstalt Helgoland, Hamburg, pp 477–961
Lee SY, Morton BS (1985) The introduction of the Mediterranean mussel Mytilus galloprovincialis into Hong Kong. Malacol Rev 18:107–109
Lesser MP, Shumway SE, Cucci T, Smith J (1992) Impact of fouling organisms on mussel rope culture: Interspecific competition for food among suspension-feeding invertebrates. J Exp Mar Biol Ecol 165(1):91–102
Liu H, Stiling P, Pemberton RW (2007) Does enemy release matter for invasive plants? evidence from a comparison of insect herbivore damage among invasive, non-invasive and native congeners. Biol Invasions 9:773–781
Lively CM (1999) Migration, virulence, and the geographic mosaic of adaptation by parasites. Am Nat 153(suppl):34–47
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the World’s Worst Invasive Alien Species: a selection from the global invasive species database. Published by The Invasive Species Specialist Group (ISSG), a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN)
Mack R, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazza F (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Miller A, Inglis GJ, Poulin R (2008) Use of the introduced bivalve, Musculista senhousia, by generalist parasites of native New Zealand bivalves. NZ J Mar Fresh 42:143–151
Mitchell CE, Power AG (2003) Release of invasive plants from fungal and viral pathogens. Nature 421:625–627
Molloy DP, Karatayev AY, Burlakova LE, Kurandina DP, Laruelle F (1997) Natural enemies of zebra mussels: predators, parasites, and ecological competitors. Rev Fish Sci 5:27–97
Mouritsen KN, Poulin R (2002) Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124:101–117
Nicastro KR, Zardi GI, McQuaid CD (2010) Differential reproductive investment, attachment strength and mortality of invasive and indigenous mussels across heterogeneous environments. Biol Invasions 12:2165–2177
Parker IM, Simberloff D, Londsale WM, Goodell KK, Wonham M, Kareiva PM, Williamson MH, Von Holles B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: Toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pasternak Z, Diamant A, Abelson A (2007) Co-invasion of a Red Sea fish and its ectoparasitic monogenean, Polylabris cf. mamaevi into the Mediterranean: observations on oncomiracidium behavior and infection levels in both seas. Parasitol Res 100:721–727
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agric Ecosyst Environ 84(1):1–20
Poulin R, Mouillot D (2003) Host introductions and the geography of parasite taxonomic diversity. J Biogeogr 30:837–845
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 7:385–390
Robinson TB, Griffiths CL, McQuaid CD, Rius M (2005) Marine alien species of South Africa: status and impacts. Afr J Mar Sci 27:297–306
Ruesink JL, Trimble AC (2010) First report of Phoronis ovalis from Africa and its effect on mussel hosts. Afr J Mar Sci 32:109–114
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Scardino A, de Nys R (2004) Fouling deterrence on the bivalve shell Mytilus galloprovincialis: a physical phenomenon? Biofouling 20:249–257
Scardino AJ, de Nys R, Ison O, O’Connor W, Steinberg PD (2003) Microtopography and antifouling properties of the shell surface of the bivalve molluscs Mytilus galloprovincialis and Pinctata imbricate. Biofouling 19(Suppl):221–230
Scardino AJ, Harvey E, De Nys R (2006) Testing attachment point theory: diatom attachment on microtextured polyimide biomimics. Biofouling 22:55–60
Scardino AJ, Guenther J, de Nys R (2008) Attachment point theory revisited: the fouling response to a microtextured matrix. Biofouling 24:45–53
Scardino AJ, Hudleston D, Peng Z, Paul NA, de Nys R (2009a) Biomimetic characterization of key surface parameters for the development of fouling resistant materials. Biofouling 25:83–93
Scardino AJ, Zhang H, Cookson DJ, Lamb RN, de Nys R (2009b) The role of nano-roughness in antifouling. Biofouling 25:757–767
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Slothbouber Galbreath JGM, Smith JE, Becnel JJ, Butlin RK, Dunn AM (2010) Reduction in post-invasion genetic diversity in Crangonyx pseudogracilis (Amphipoda: Crustacea): a genetic bottleneck or the work of hitchhiking vertically transmitted microparasites? Biol Invasions 12:191–209
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Suarez AV, Holway DA, Ward PS (2005) The role of opportunity in the unintentional introduction of nonnative ants. Proc Natl Acad Sci USA 102:17032–17035
Tolman HL (1999) User manual and system documentation of WAVEWATCH-III version 1.18, 110p, N.O.A.A., National Centers for Environmental Prediction. OMB Technical Note 166. Camp Springs, MD, USA
Tolman HL (2002) User manual and system documentation of WAVEWATCH-III version 2.22. 133p., Technical Note, N.O.A.A., National Centers for Environmental Prediction. OMB Technical Note 222. Camp Springs, MD, USA
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2:183–190
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3:333–345
Torchin ME, Lafferty KD, Kuris AM (2002) Parasites and marine invasions. Parasitology 124:137–151
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
Tribollet A (2007) Dissolution of dead corals by euendolithic microorganisms across the Northern Great Barrier Reef (Australia). Microb Ecol 55:569–580
Tribollet A (2008) The boring microflora in modern coral reef ecosystems: a review of its roles. In: Wissak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 67–94
Underwood AJ, Chapman MG and Richards SA (2002) GMAV-5 for Windows. An analysis of variance programme. Centre for Research on Ecological Impacts of Coastal Cities. Marine Ecology Laboratories, University of Sydney, Australia
van Erkom Schurink C, Griffiths CL (1991) A comparison of reproductive cycles and reproductive output in four southern African mussel species. Mar Ecol Prog Ser 76:123–134
Wahl M (1997) Increased drag reduces growth of snails: comparison of flume and in situ experiments. Mar Ecol Prog Ser 151:291–293
Wahl M (2008) Ecological lever and interface ecology: epibiosis modulates the interactions between host and environment. Biofouling 24:427–438
Wahl M, Hay ME (1995) Associational resistance and shared doom: effects of epibiosis on herbivory. Oecologia 102:329–340
Walker JJ, Pace NR (2007) Phylogenetic composition of rocky mountain endolithic microbial ecosystems. Appl Environ Microbiol 11:3497–3504
Wattier RA, Haine ER, Beguet J, Martin G, Bollache L, Muskó IB, Platvoet D, Rigaud T (2007) No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos 116:1941–1953
Webb SC, Korrûbel JL (1994) Shell weakening in marine mytilids attributable to blue-green alga, Mastigocoleus sp. (Nosto-chopsidaceae). J Shellfish Res 13:11–17
Wilkens NP, Fujino K, Gosling EM (1983) The Mediterranean mussel Mytilus galloprovincialis Lmk. In Japan. Biol J Linn Soc 20:365–374
Wolfe LM (2002) Why alien invaders succeed : support for the escape-from-enemy hypothesis. Am Nat 160:705–711
Wonham MJ (2004) Mini-review: distribution of the Mediterranean mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) and hybrids in the Northeast Pacific. J Shellfish Res 23:535–543
Zardi GI, Nicastro KR, Porri F, McQuaid CD (2006) Sand stress as a non-determinant of habitat segregation of indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels in South Africa. Mar Biol 148:1031–1038
Zardi GI, McQuaid CD, Nicastro KR (2007) Balancing survival and reproduction: seasonality of wave action, attachment strength and reproductive output in indigenous Perna perna and invasive Mytilus galloprovincialis mussels. Mar Ecol Prog Ser 334:155–163
Zardi GI, Nicastro KR, McQuaid CD, Erlandsson J (2008) Sand and wave induced mortality in invasive (Mytilus galloprovincialis) and indigenous (Perna perna) mussels. Mar Biol 153:853–858
Zardi GI, Nicastro KR, McQuaid CD, Gektidis M (2009) Effects of endolithic parasitism on invasive and indigenous mussels in a variable physical environment. PLoS ONE 8:1–10
Acknowledgments
This research was supported by post-doctoral fellowships from FCT, Portugal (to GIZ) and funded by project PTDC/BIA-BEC/103916/2008 from FCT (to GIZ). The authors thank C. Florindo (Universidade do Algarve, Cell Imaging Unit; Dept. de Ciencias Biomédicas e Medicina) for her help with preparing samples and microscopy image analysis, and A. Morgado-André and F. Oliveira (Universidade do Algarve, Civil Engineering Department, Escola Superior de Tecnologia) for their assistance in the use of the compression equipment. This work is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation. We thank also two anonymous referees for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10530_2012_363_MOESM2_ESM.pptx
Figure 1S. Examples of shells at varying stages of endolith infestation. Shells with clean, intact periostracum and distinct outer growth lines (Group A); shells with central portion of surface eroding and turning pale purple, outer growth lines becoming indistinct (Group B); shells with erosion spreading past central portion, grooves and pits appearing on shell surface (Group C); shells heavily pitted and becoming deformed, outer growth lines almost completely absent (Group D); shells extremely pitted, deformed and brittle, eventually holed (Group E). Scale bar = 1cm. (PPTX 114 kb)
10530_2012_363_MOESM3_ESM.tif
Figure 2S. Microbial endoliths of M. galloprovincialis. Euendoliths depicted in this plate from A to D exhibit a specific habitus, which allows a taxonomic classification to genus level. Euendoliths depicted from E to F were not found in sufficient numbers or did not exhibit typical habitus for a definite taxonomic identification. (A - D) Euendoliths of cyanobacterial origin and members of the genus Hyella. In (A) arrow points to an elongated terminal cell. Filaments are long and cells arranged in a single row. Terminal cell always much longer than subterminal cell. In (B) arrow points to a large cluster of baeocytes, which gives the colony an arachnoid appearance. (E - F) Unidentified euendoliths of cyanobacterial origin. Habitus and cell size place this species close to the genus Solentia. The red colour is maintained next to green coloured cells of another species. This and the large cell size place this species very close to Solentia sanguina. However, neither the very prominent layered sheath, nor the frequent ramification of S. sanguina were observed in this colony. In (E) arrow points to a terminal cell. In (F) arrow points to the enhanced distance between terminal and subterminal cell. (TIFF 11988 kb)
10530_2012_363_MOESM4_ESM.tif
Figure 3S. Microbial endoliths of M. galloprovincialis. Euendoliths depicted in this plate were not found in sufficient numbers or did not exhibit typical habitus for a definite taxonomic identification. (A) Heterotrophic endolith, presumably of fungal origin. Arrow points to tunnel entrance. (B) Euendolith of cyanobacterial origin. Habitus and cell size place this organism close to the genus Hyella or Solentia. Arrow points to barely visible gelatinous sheath. Terminal cells are elongated and at a greater distance to subterminal cells, which is characteristic of Solentia. (C) Euendolith of cyanobacterial origin. Habitus and cell size place this species close to Hyella or Cyanosaccus. Hyella is more likely, because at some points, the cell number exceeds four. Baeocytes are packed as in Hyella immanis or H. conferta. Arrow shows retouched area, where the original scale had been burned into the picture and then manually removed from the picture. (D) Euendolith of cyanobacterial origin. Habitus and cell size place this species close to Hyella or Solentia. Pink colour might be an artifact or pigmentation like with Solentia sanguina but this species is much smaller than S. sanguina (CL ~24µm CW~14 µm). Arrow points to elongated terminal cell. (E) Euendolith of cyanobacterial origin. Habitus and cell size place this species close to Hyella. Baeocytes are packed as in Hyella immanis or H. conferta. (F) Euendolith of cyanobacterial origin. Habitus and cell size place this species close to Hyella. Arrow points to vegetative cells surrounded by sheath. (TIFF 10709 kb)
Rights and permissions
About this article
Cite this article
Marquet, N., Nicastro, K.R., Gektidis, M. et al. Comparison of phototrophic shell-degrading endoliths in invasive and native populations of the intertidal mussel Mytilus galloprovincialis . Biol Invasions 15, 1253–1272 (2013). https://doi.org/10.1007/s10530-012-0363-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0363-1