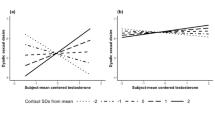
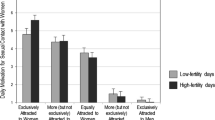
Abstract
Although many studies report that women’s sexual behavior varies across their menstrual cycles, the research findings remain inconsistent. In this study, we addressed two methodological issues in research on the menstrual cycle: how ovulation is measured/inferred and whether data using menstrual cycles or participants’ scores averaged across cycles as units of analysis yield similar results. We also employed an abstinent comparison group in addition to examining how emotional well-being was related to libido and sexual behavior through factor and regression analysis. Data were obtained from 97 participants. There were no significant differences in the results of analyses performed using cycles with known LH surges to determine ovulation versus cycles based on backward counts. However, we concluded that statistical power might be compromised when the known timing of ovulation was less accurate. Likewise, we found few overall differences in the results when we analyzed data using cycles with known LH surges compared to participants’ averaged data across cycles. Women, including those in the abstinent group, reported increased sexual behavior prior to ovulation. Allosexual behavior was positively related to libido, and negatively related to positive and “premenstrual” emotional factors. Autosexual behavior was predicted by libido and an energetic/creative emotional factor. Our findings support hypotheses that women’s sexual behavior is related to both mating and pair-bond formation.
Similar content being viewed by others
References
Adams, D. B., Gold, A. R., & Burt, A. D. (1978). Rise in female-initiated sexual arousal at ovulation and its suppression by oral contraceptive. New England Journal of Medicine, 299, 1145–1150.
Aron, A., Fisher, H. E., Mashek, D. J., Strong, G., Li, H., & Brown, L. L. (2005). Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94, 327–337. doi:10.1152/jn.00838.2004.
Bancroft, J., Sanders, D., Davidson, D., & Warner, P. (1983). Mood, sexuality, hormones, and the menstrual cycle III. Sexuality and the role of androgens. Psychosomatic Medicine, 45, 509–516.
Bartels, A., & Zeki, S. (2004). The neural correlates of maternal and romantic love. NeuroImage, 21, 1155–1166. doi:10.1016/j.neuroimage.2003.11.003.
Bellis, M. A., & Baker, R. R. (1990). Do females promote sperm competition? Data for humans. Animal Behaviour, 40, 997–999. doi:10.1007/978-0-387-28039-4_9.
Brewis, A., & Meyer, M. (2005). Demographic evidence that human ovulation is undetectable (at least in pair bonds). Current Anthropology, 46, 465–471. doi:10.1086/430016.
Brown, S. G., Morrison, L. A., Calibuso, M. J., & Christiansen, T. M. (2008a). The menstrual cycle and sexual behavior: Relationship to eating, exercise, sleep, and health patterns. Women and Health, 48, 429–444. doi:10.1080/03630240802575179.
Brown, S. G., Morrison, L. A., Larkspur, L. M., Marsh, A. L., & Nicolaisen, N. (2008b). Well-being, sleep, exercise patterns, and the menstrual cycle: A comparison of natural hormones, oral contraceptives and Depo-Provera. Women and Health, 47, 105–121. doi:10.1300/J013v47n01_06.
Bullivant, S. B., Sellergren, S. A., Stern, K., Spencer, N. A., Jacob, S., Mennella, J. A., et al. (2004). Women’s sexual experience during the menstrual cycle: Identification of the sexual phase by noninvasive measurement of luteinizing hormone. Journal of Sex Research, 41, 82–93.
Burleson, M. H., Trevathan, W. R., & Gregory, W. L. (2002). Sexual behavior in lesbian and heterosexual women: Relations with menstrual cycle phase and partner availability. Psychoneuroendocrinology, 27, 489–503. doi:10.1016/S0306-4530(01)00066-X.
Dekker, A., & Schmidt, G. (2002). Patterns of masturbatory behavior: Changes between the sixties and the nineties. Journal of Psychology & Human Sexuality, 14, 35–48. doi:10.1300/J056v14n02_04.
Diamond, L. M. (2003). What does sexual orientation orient? A biobehavioral model distinguishing romantic love and sexual desire. Psychological Review, 110, 173–192. doi:10.1037/0033-295X.110.1.173.
Englander-Golden, P., Chang, H., Whitmore, M. R., & Dienstbier, R. A. (1980). Female sexual arousal and the menstrual cycle. Journal of Human Stress, 6, 42–48.
Ferin, M., Jewelewicz, R., & Warren, M. (1993). The menstrual cycle: Physiology, reproductive disorders, and infertility. New York: Oxford University Press.
Fisher, H. E., Aron, A., & Brown, L. L. (2006). Romantic love: A mammalian brain system for mate choice. Philosophical Transactions of the Royal Society B, 361, 2173–2186. doi:10.1098/rstb.2006.1938.
Fisher, H. E., Aron, A., Mashek, D., Li, H., & Brown, L. L. (2002). Defining the brain systems of lust, romantic attraction, and attachment. Archives of Sexual Behavior, 31, 413–419. doi:10.1023/A:1019888024255.
Gangestad, S. W., & Thornhill, R. (2008). Human oestrus. Proceedings of the Royal Society London B, 275, 991–1000. doi:10.1098/rspb.2007.1425.
Gangestad, S. W., Thornhill, R., & Garver, C. E. (2002). Changes in women’s sexual interests and their partners’ mate-retention tactics across the menstrual cycle: Evidence for shifting conflicts of interest. Proceedings of the Royal Society of London B, 269, 975–982. doi:10.1098/rspb.2001.1952.
Garver-Apgar, C. E., Gangestad, S. W., & Thornhill, R. (2008). Hormonal correlates of women’s mid-cycle preference for the scent of symmetry. Evolution and Human Behavior, 29, 223–232. doi:10.1016/j.evolhumbehav.2007.12.007.
Gonzaga, G. C., Turner, R. A., Keltner, D., Campos, B., & Altemus, M. (2006). Romantic love and sexual desire in close relationships. Emotion, 6, 163–179. doi:10.1037/1528-3542.6.2.163.
Graham, C. A., Janssen, E., & Sanders, S. A. (2000). Effects of fragrance on female sexual arousal and mood across the menstrual cycle. Psychophysiology, 37, 78–84. doi:10.1017/S0048577200981964.
Harvey, S. (1987). Female sexual behavior: Fluctuations during the menstrual cycle. Journal of Psychosomatic Research, 31, 101–110.
Haselton, M. G., & Gangestad, S. W. (2006). Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior, 49, 509–518. doi:10.1016/j.yhbeh.2005.10.006.
Haselton, M. G., Mortezaie, M., Pillsworth, E. G., Bleske-Rechek, A., & Frederick, D. A. (2007). Ovulation and human female ornamentation: Near ovulation, women dress to impress. Hormones and Behavior, 51, 40–45. doi:10.1016/j.yhbeh.2006.07.007.
Hill, E. M. (1988). The menstrual cycle and components of human female sexual behaviour. Journal of Social and Biological Structures, 11, 443–455. doi:10.1016/0140-1750(88)90082-6.
Hourani, L. L., Yuan, H., & Bray, R. M. (2004). Psychosocial and lifestyle correlates of premenstrual symptoms among military women. Journal of Women’s Health, 13, 812–821. doi:10.1089/jwh.2004.13.812.
Kontula, O., & Haavio-Mannila, E. (2003). Masturbation in a generational perspective. Journal of Psychology & Human Sexuality, 14, 49–83. doi:10.1300/J056v14n02_05.
Krug, R., Plihal, W., Fehm, H. L., & Born, J. (2000). Selective influence of the menstrual cycle on perception of stimuli with reproductive significance: An event-related potential study. Psychophysiology, 37, 111–122. doi:10.1017/S0048577200980594.
Laeng, B., & Falkenberg, L. (2007). Women’s pupillary responses to sexually significant others during the hormonal cycle. Hormones and Behavior, 52, 520–530. doi:10.1016/j.yhbeh.2007.07.013.
Matteo, S., & Rissman, E. F. (1984). Increased sexual activity during the midcycle portion of the human menstrual cycle. Hormones and Behavior, 18, 249–255. doi:10.1016/0018-506X(84)90014-X.
Miller, G., Tybur, J. M., & Jordan, B. D. (2007). Ovulatory cycle effects on tip earnings by lap dancers: Economic evidence for human estrus? Evolution and Human Behavior, 28, 375–381. doi:10.1016/j.evolhumbehav.2007.06.002.
Phan, K. L., Wager, T., Taylor, S. F., & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16, 331–348. doi:10.1006/nimg.2002.1087.
Pillsworth, E. G., Haselton, M. G., & Buss, D. M. (2004). Ovulatory shifts in female sexual desire. Journal of Sex Research, 41, 55–65.
Pipitone, R. N., & Gallup, G. G. (2007). Women’s voice attractiveness varies across the menstrual cycle. Evolution and Human Behavior, 29, 268–274. doi:10.1016/j.evolhumbehav.2008.02.001.
Roberts, S. C., Havlicek, J., Flegr, J., Hruskova, M., Little, A. C., Jones, B. C., et al. (2004). Female facial attractiveness increases during the fertile phase of the menstrual cycle. Proceedings of the Royal Society London B, 271(Suppl.), S270–S272. doi:10.1098/rsbl.2004.0174.
Roney, J. R., & Simmons, Z. L. (2008). Women’s estradiol predicts preference for facial cues of men’s testosterone. Hormones and Behavior, 53, 14–19. doi:10.1016/h.yhbeh.2007.09.008.
Rosen, M. L., & Lopez, H. H. (2009). Menstrual cycle shifts in attentional bias for courtship language. Evolution and Human Behavior, 30, 131–140. doi:10.1016/j.evolhumbehav.2008.09.007.
Sanders, D., Warner, P., Backstrom, T., & Bancroft, J. (1983). Mood, sexuality, hormones and the menstrual cycle I. Changes in mood and physical state: Description of subjects and method. Psychosomatic Medicine, 45, 487–501.
Schreiner-Engel, P., Schaivi, R. C., Smith, H., & White, D. (1981). Sexual arousability and the menstrual cycle. Psychosomatic Medicine, 43, 199–214.
Schwarz, S., & Hassebrauck, M. (2008). Self-perceived and observed variations in women’s attractiveness throughout the menstrual cycle—A diary study. Evolution and Human Behavior, 29, 282–288. doi:10.1016/j.evolhumbehav.2008.02.003.
Sheldon, M. S., Cooper, M. L., Geary, D. C., Hoard, M., & DeSoto, M. C. (2006). Fertility cycle patterns in motives for sexual behavior. Personality and Social Psychology Bulletin, 32, 1659–1673. doi:10.1177/0146167206292690.
Siiteri, P. K. (1987). Adipose tissue as a source of hormones. American Journal of Clinical Nutrition, 45, 277–282.
Singh, D., & Bronstad, P. M. (2001). Female body odour is a potential cue to ovulation. Proceedings of the Royal Society London B, 268, 797–801. doi:10.1098/rspb.2001.1589.
Slob, A. K., Bax, C. M., Hop, W. C. J., Rowland, D. L., & ten Bosch, J. J. (1996). Sexual arousability and the menstrual cycle. Psychoneuroendocrinology, 21, 545–558. doi:10.1016/0306-4530(95)00058-5.
Stanislaw, H., & Rice, E. J. (1988). Correlation between sexual desire and menstrual cycle characteristics. Archives of Sexual Behavior, 17, 499–508.
van Goozen, S. H. M., Wiegant, V. M., Endert, E., & Helmond, E. A. (1997). Psychoendocrinological assessment of the menstrual cycle: The relationship between hormones, sexuality, and mood. Archives of Sexual Behavior, 26, 359–382.
Walker, A. E. (1997). The menstrual cycle. New York: Routledge.
Walter, M., Bermpohl, F., Mouras, H., Schiltz, K., Tempelmann, C., Rotte, M., et al. (2008). Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. NeuroImage, 40, 1482–1494. doi:10.1016/j.neuroimage.2008.01.040.
Wilcox, A. J., Baird, D. D., Dunson, D. B., McConnaughey, D. R., Kesner, J. S., & Weinberg, C. R. (2004). On the frequency of intercourse around ovulation: Evidence for biological influences. Human Reproduction, 19, 1539–1543. doi:10.1093/humrep/deh305.
Wilcox, A. J., Weinberg, C. R., & Baird, D. D. (1995). Timing of sexual intercourse in relation to ovulation—Effects on the probability of conception, survival of the pregnancy, and sex of the baby. New England Journal of Medicine, 333, 1517–1521.
Acknowledgements
We thank Marci Arizumi, Louona Larkspur, Ariel Marsh, Lynn Morrison, Nicola Nicolaisen, Tiffany Freitas, Michelle McNamee, Sarah Asakawa, Aizelle Boado, and Lisa Oliver in helping to recruit the participants, and in data collection, organization, and analysis. This research was supported by NIH grant #S06-GM0873-33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, S.G., Calibuso, M.J. & Roedl, A.L. Women’s Sexuality, Well-Being, and the Menstrual Cycle: Methodological Issues and Their Interrelationships. Arch Sex Behav 40, 755–765 (2011). https://doi.org/10.1007/s10508-010-9630-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-010-9630-3