Abstract
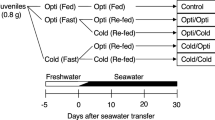
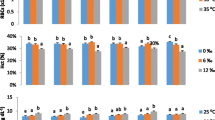
The aim of this study was to reveal possible interactive effects of temperature and photoperiod on somatic and skeletal growth, feed conversion, organ indexes and blood chemistry in Atlantic salmon postsmolts. A total of 1140 (initial mean weight 96.0 g ± 3.1 SEM) juvenile Atlantic salmon reared in seawater were in duplicates exposed to six different combinations of temperatures (4.3, 6.5 or 9.3 °C) and photoperiods (continuous light, LL or simulated natural photoperiod (69ºN), LDN) for 124 days. An interactive effect of photoperiod and temperature on somatic growth was found as the fish exposed to low temperature and continuous light regime (4LL) had a significantly higher growth (30 % gain in overall SGR) than the 4LDN group, corresponding to the effect of approx. 1.2 °C temperature increase. Fish in the 6 and 9 °C groups did not show any significant growth benefit of continuous light. Compared to the 4LDN group, the 4LL group showed higher total feed conversion efficiency, lower levels of blood Na+ and lower hepato-somatic and cardio-somatic indexes. In the skeleton, cervical vertebra were largest in the 4LL group, while the length of the head was largest in the 4LDN group, continuous light promotes growth at lower temperatures while supporting a normal development. It is suggested that a considerable growth benefit may be achieved by exposing juvenile Atlantic salmon to continuous light when reared at low (in this trial 4.3 °C) water temperature during winter.








Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- LL:
-
Continuous light
- LDN:
-
Simulated natural photoperiod
- SGR:
-
Specific growth rate
References
Aas Ø, Klemetsen A, Einum S, Skurdal J (2011) Atlantic salmon ecology. Wiley-Blackwell, West Sussex
Arnesen AM, Johnsen HK, Mortensen A, Jobling M (1998) Acclimation of Atlantic salmon (Salmo salar L.) smolts to ‘cold’ sea water following direct transfer from fresh water. Aquaculture 168:351–367. doi:10.1016/s0044-8486(98)00361-5
Boeuf G, Le Bail PY (1999) Does light have an influence on fish growth? Aquaculture 177:129–152. doi:10.1016/S0044-8486(99)00074-5
Bolton JP, Young G, Nishioka RS, Hirano T, Bern HA (1987) Plasma growth hormone levels in normal and stunted yearling coho salmon, Oncorhynchus kisutch. J Exp Zool 242:379–382
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98. doi:10.1016/s0044-8486(01)00583-x
Brown MB, Forsythe AB (1974) Robust tests for the equality of variances. J Am Stat Assoc 69:364–367. doi:10.1080/01621459.1974.10482955
Clarke WC, Shelbourn JE, Brett JR (1978) Growth and adaptation to sea water in ‘underyearling’ sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon subjected to regimes of constant or changing temperature and day length. Can J Zool 56:2413–2421
Cnaani A, McLean E, Hallerman EM (2013) Effects of growth hormone transgene expression and triploidy on acute stress indicators in Atlantic salmon (Salmo salar L.). Aquaculture 412–413:107–116. doi:10.1016/j.aquaculture.2013.06.029
Fjelldal PG, Nordgarden U, Berge A, Grotmol S, Totland GK, Wargelius A, Hansen T (2005) Vertebrae of the trunk and tail display different growth rates in response to photoperiod in Atlantic salmon, Salmo salar L., post-smolts. Aquaculture 250:516–524
Fjelldal PG, Hansen T, Breck O, Sandvik R, Waagbø R, Berg A, Ørnsrud R (2009) Supplementation of dietary minerals during the early seawater phase increase vertebral strength and reduce the prevalence of vertebral deformities in fast growing under-yearling Atlantic salmon (Salmo salar L.) smolt. Aquac Nutr 15:366–378
Grini A, Hansen T, Berg A, Wargelius A, Fjelldal PG (2011) The effect of water temperature on vertebral deformities and vaccine-induced abdominal lesions in Atlantic salmon, Salmo salar L. J Fish Dis 34:531–546
Handeland SO, Berge A, Bjornsson BTh, Lie O, Stefansson SO (2000) Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 181:377–396. doi:10.1016/s0044-8486(99)00241-0
Handeland SO, Th Björnsson B, Arnesen AM, Stefansson SO (2003) Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture 220:367–384. doi:10.1016/S0044-8486(02)00508-2
Handeland SO, Imsland AK, Stefansson SO (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283:36–42
Hosfeld CD, Hammer J, Handeland SO, Fivelstad S, Stefansson SO (2009) Effects of fish density on growth and smoltification in intensive production of Atlantic salmon (Salmo salar L.). Aquaculture 294:236–241. doi:10.1016/j.aquaculture.2009.06.003
Houde ED, Schekter RC (1981) Growth rates, rations and cohort consumption of marine fish larvae in relation to prey concentrations. Rapp Proc-verb Réun Con inter l’Explor Mer 178:441–453
Hovland E, Møller D (2010) Åkeren kan òg være blå. Et riss av havbruksnæringens utvikling i Norge (The field can also be blue. The history of aquaculture in Norway). Norwegian Directory of Fisheries, Bergen, Norway, ISBN 978-82-93011-06-4
Hutchings JA, Jones MEB (1998) Life history variation and growth rate thresholds for maturity in Atlantic salmon, Salmo salar. Can J Fish Aquat Sci 55:22–47. doi:10.1139/d98-004
Imsland AK, Jonassen TM (2001) Regulation of growth in turbot (Scophthalmus maximus Rafinesque) and Atlantic halibut (Hippoglossus hippoglossus L.): aspects of environment x genotype interactions. Rev Fish Biol Fish 11:71–90. doi:10.1023/a:1014240430779
Imsland AK, Folkvord A, Stefansson S (1995) Growth, oxygen consumption and activity of juvenile turbot (Scophthalmus maximus) reared under different temperatures and photoperiods. Neth J Sea Res 34:149–159
Imsland A, Handeland S, Stefansson SO (2014) Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquac Int 22:1331–1345. doi:10.1007/s10499-014-9750-1
Jonassen TM, Imsland AK, Kadowaki S, Stefansson SO (2000) Interaction of temperature and photoperiod on growth of Atlantic halibut, Hippoglossus hippoglossus L. Aquac Res 31:219–227
Kacem A, Meunier FJ, Bagliniere JL (1998) A quantitative study of morphological and histological changes in the skeleton of Salmo salar during its anadromous migration. J Fish Biol 53:1096–1109
Koskela J, Pirhonen J, Jobling M (1997) Effect of low temperature on feed intake, growth rate and body composition of juvenile Baltic salmon. Aquac Int 5:479–488. doi:10.1023/A:1018397014684
Kråkenes R, Hansen T, Stefansson SO, Taranger GL (1991) Continuous light increases growth rate of Atlantic salmon (Salmo salar L.) postsmolts in sea cages. Aquaculture 95:281–287. doi:10.1016/0044-8486(91)90093-M
McAllister KW, McAllister PE, Simon RC, Werner JK (1992) Performance of nine external tags on hatchery-reared rainbow trout. Trans Am Fish Soc 121:192–198
McCormick SD, Saunders RL, Henderson EB, Harmon PR (1987) Photoperiod control of parr-smolt transformation in Atlantic salmon (Salmo salar): changes in salinity tolerance, gill Na+, K+-ATPase activity, and plasma thyroid hormones. Can J Fish Aquat Sci 44:1462–1468
Norberg B, Weltzien FA, Karlsen Ø, Holm JC (2001) Effects of photoperiod on sexual maturation and somatic growth in male Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Physiol Part B Biochem Mol Biol 129:357–365
Nordgarden U, Oppedal F, Taranger GL, Hemre GI, Hansen T (2003) Seasonally changing metabolism in Atlantic salmon (Salmo salar L.) I—Growth and feed conversion ratio. Aquac Nutr 9:287–293
Oppedal F, Berg A, Olsen RE, Taranger GL, Hansen T (2006) Photoperiod in seawater influence seasonal growth and chemical composition in autumn sea-transferred Atlantic salmon (Salmo salar L.) given two vaccines. Aquaculture 254:396–410
Pettersen JM, Bracke MBM, Midtlyng PJ, Folkedal O, Stien LH, Steffenak H, Kristiansen TS (2014) Salmon welfare index model 2.0: an extended model for overall welfare assessment of caged Atlantic salmon, based on a review of selected welfare indicators and intended for fish health professionals. Rev Aquac 6:162–179. doi:10.1111/raq.12039
Rosenfeld J, Van Leeuwen T, Richards J, Allen D (2015) Relationship between growth and standard metabolic rate: measurement artefacts and implications for habitat use and life-history adaptation in salmonids. J Anim Ecol 84:4–20. doi:10.1111/1365-2656.12260
Roth B, Johansen SJS, Suontama J, Kiessling A, Leknes O, Guldberg B, Handeland S (2005) Seasonal variation in flesh quality, comparison between large and small Atlantic salmon (Salmo salar) transferred into seawater as 0+ or 1+ smolts. Aquaculture 250:830–840. doi:10.1016/j.aquaculture.2005.05.009
Stefansson SO, Bjornsson BT, Hansen T, Haux C, Taranger GL, Saunders RL (1991) Growth, parr-smolt transformation, and changes in growth-hormone of Atlantic salmon (Salmo-salar) reared under different photoperiods. Can J Fish Aquat Sci 48:2100–2108
Stefansson SO, Nilsen TO, Ebbesson LOE, Wargelius A, Madsen SS, Bjornsson BT, McCormick SD (2007) Molecular mechanisms of continuous light inhibition of Atlantic salmon parr-smolt transformation. Aquaculture 273:235–245. doi:10.1016/j.aquaculture.2007.10.005
Treasurer J, Atack T, Rolton A, Walton J, Bickerdike R (2011) Social, stocking density and dietary effects on the failure of farmed cod Gadus morhua. Aquaculture 322–323:241–248
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, Englewood Cliffs
Acknowledgments
Financial support was given by the Research Council of Norway (RFFNord, Contract: 226059 NORDLYS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Døskeland, I., Imsland, A.K.D., Fjelldal, P.G. et al. The effect of low temperatures and photoperiods on growth and vertebra morphometry in Atlantic salmon. Aquacult Int 24, 1421–1434 (2016). https://doi.org/10.1007/s10499-016-9999-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-9999-7