Abstract
Healthy Labeo rohita (mean bodyweight of 20.1 g) were divided into four groups before being fed for 60 days on diets supplemented with 0 (control), 20 (E1), 30 (E2), or 40 (E3) mg kg−1 emodin. Various growth and immune parameters were measured after 15, 30, 45, and 60 days of feeding. Fish fed E2 diet exhibited accelerated (p < 0.05) weight gain after 30–60 days of feeding. The most significant improvements (p < 0.05) in immune parameters, such as lysozyme activity, alternative complement pathway activity, respiratory bursts activity, phagocytic activity, superoxide dismutase activity, and myloperoxidase activity, were observed in the E2-fed group after 30 and 45 days of feeding. However, fish groups fed E2 or E3 diets exhibited significantly lower malondialdehyde, aspartate aminotransferase, and alanine aminotransferase activities than did the control group after 30 and 45 days of feeding. The IgM level was significantly elevated in treatment groups after 30 and 45 days of feeding. Further, fish fed E2 diet for 45 days had the highest (p < 0.005) post-challenge survival rate (83.3 %), followed by fish fed E2 diet for 30 days (75 %). Therefore, dietary feeding of emodin at 30 mg kg−1 to L. rohita for 30–45 days is optimal to enhance the immunity and disease resistance against A. hydrophila.
Similar content being viewed by others
Introduction
Freshwater fish dominate global aquaculture production (56.4 %), with carp being the most commonly raised species (71.9 %; 24.2 million tons in 2010) (FAO 2012). Indian aquaculture mainly consists (~70 %) of three carp species: Labeo rohita, Catla catla, and Cirrhinus mrigala (FAO 2012). In intensive aquaculture, fish often encounter high temperatures, poor water quality, and overcrowding. These conditions result in poor physiological environments, which increase the susceptibility of fish to infections (Ming et al. 2012). Disease outbreaks are potential constraints on aquaculture and cause huge economic losses and reduced profit margins through mortality or inferior meat quality (Smith et al. 2003). The most frequently encountered bacterial pathogen, Aeromonas hydrophila, severely damages carp production (Giri et al. 2013). Traditionally, antibiotics, vaccines, and chemotherapeutics have been used for disease control. However, the use of antibiotics for disease control leads to the development of drug-resistant pathogens, environmental hazards, and food safety problems (Austin and Austin 2007). Moreover, A. hydrophila is a heterogeneous species with variable antigens, which makes vaccine development extremely difficult (Yin et al. 2009). Vaccines against specific pathogens have been developed with varying degrees of success, but the wide range of pathogens in fish farming limits the practicality of vaccines (Harikrishnan et al. 2011). Therefore, there is an urgent need to develop natural or eco-friendly therapeutics, such as immune-stimulants, probiotics, and therapeutics from plants (Liu et al. 2012).
Several herbs are being used as therapeutic agents for controlling diseases in aquaculture. A number of materials and products from species including Astragalus radix, Ganoderma lucidum (Yin et al. 2009), Rheum officinale (Xie et al. 2008; Liu et al. 2012), Allium sativum (Sahu et al. 2006), Withania somnifera (Sharma et al. 2010), Styrax japonica (Harikrishnan et al. 2011), and Achyranthes aspera (Rao et al. 2006; Sheikhzadeh et al. 2012) have been reported to enhance the immunity of fish against diseases.
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone), a medicinal extract present in many herbs such as Aloe barbadensis, R. officinale, Cassia angustifolia, Polygonum multiflorum, and Polygonum cuspidatum, has been used as traditional medicine in eastern Asia and shows promise as an immunostimulant (Zhang et al. 2014a). Heo et al. (2008) demonstrated that emodin inhibits tumor necrosis factor-alpha (TNF-α)-induced human aortic smooth-muscle cell (HASMC) proliferation via caspase- and mitochondrial-dependent apoptotic pathways. Their results indicate that emodin has potential as an anti-atherosclerosis agent. Many properties have been reported for emodin, such as anti-bacterial activity against A. hydrophila (Zhang et al. 2014b), anti-inflammatory effects against carrageenan-induced edema in rats (Chang et al. 1996), hepatoprotective ability against acetaminophen-induced toxicity in albino rats (Bhadauria 2010), and regulation of immune responses in human mesangial cells (Kuo et al. 2001). A few investigations have demonstrated the effects of emodin on growth and immune responses in aquatic animals. In Wuchang bream (Megalobrama amblycephala), dietary supplementation with emodin upregulated the expression of two HSP70s mRNA and promoted growth, non-specific immunity, and antioxidant capacity (Ming et al. 2012). Supplementation with 1–2 % anthraquinone extract promotes growth, mitigates the negative effects of stress due to crowding, and enhances the resistance against pathogenic infection in Cyprinus carpio var. Jian (Xie et al. 2008). However, there is no report regarding the effects of emodin on the growth, immune responses, and disease resistance of the major carps in Indian aquaculture.
The effect of oral immunostimulants on the immune response depends on the dose of the immunostimulant and the duration with which it is fed to the aquatic animals (Zhang et al. 2014a). Therefore, the present study was designed to investigate the effects of dietary supplementation with different doses of emodin on the growth performance and immune parameters of L. rohita fingerlings. Resistance to A. hydrophila infection of L. rohita fingerlings fed emodin-supplemented diets was also investigated.
Materials and methods
Diet preparation
Basal diet preparation is described in Giri et al. (2012). Proximate analysis (AOAC 1997) of the basal diet revealed a composition of 37.1 % protein, 8.7 % lipid, and 12 % ash. The basal diet was considered the control diet. Three experimental diets (E1, E2, and E3) were prepared with the addition of 20, 30, and 40 mg kg−1 emodin, respectively, which was extracted from Frangula bark (Sigma-Aldrich, USA). All ingredients were mixed, blended thoroughly, pelleted, air-dried, ground, and sieved into proper pellet size. All feed was stored at −20 °C until use.
Experimental design
L. rohita fingerlings (mean bodyweight: 20.1 ± 0.07 g) were obtained from Mannal freshwater fish farm, Thanjavur, Tamil Nadu, and acclimatized to laboratory conditions for 2 weeks in 500 l plastic quarantine tanks at 27 ± 2 °C. Fish were fed with basal diet during the acclimatization period. About 20 % of the water in all tanks was exchanged daily, and 100 % of the water was exchanged once a week. Basic physiochemical parameters of the water were measured every week (APHA, AWWA, WEF 1998). The O2 and ammonia concentrations ranged from 6.1 to 7.3 mg l−1 and 0.03–0.06 mg l−1 ppm, respectively, and pH ranged from 7.0 to 8.0 throughout the study period.
Fish were randomly divided into four experimental groups across 12 tanks (3 tanks/group; 200 l water/tank). Each tank was stocked with 50 fish. Fish were fed with one of the four diets (basal diet, E1, E2, or E3) for 60 days. Fish were fed thrice per day (7:00, 12:00, and 18:00) at the rate of 2–4 % of body weight. The amount of feed consumed was determined by daily recovery of excess feed, and the amount of feed provided was adjusted every 15 days by batch weighing after 24 h of starvation (Sun et al. 2010).
Growth performance
Five fish were randomly selected from each tank (i.e., 5 fish × 3 tanks = 15 fish per group) after 0, 15, 30, 45, and 60 days of experimental feeding and batch weighed to estimate growth performance. Growth performance was calculated using the following formulas:
where t is the duration of feeding (in days), FI is feed intake and W t and W 0 are final and initial fish weight, respectively.
Sample collection
Sampling occurred after 15, 30, 45, and 60 days of experimental feeding. For each sample, three fish were collected randomly from each tank (i.e., 3 fish × 3 tanks = 9 fish per group) for immunological assays. Blood samples were collected by caudal venipuncture using a 1-ml syringe after anesthetizing the fish with diluted MS222 (Sigma-Aldrich, USA). Blood samples were transferred into Eppendorf tubes and centrifuged at 2000×g for 10 min at 4 °C. Obtained blood leukocytes and plasma were stored at −20 °C for further analysis.
Head kidney macrophages were isolated from nine fish per dietary group using the method of Secombes (1990) with the modifications made by Geng et al. (2012). Harvested cells were adjusted to 1 × 107 cells ml−1 for the assay.
Immunological assays
Lysozyme activity
Lysozyme activity (LA) was measured according to the method described by Ellis (1999). One unit of LA was defined as the amount of enzyme required to decrease absorbance by 0.001 min−1 ml−1 serum.
Alternative complement pathway (ACP) activity
ACP activity (ACH50) was determined and calculated using the method of Yano et al. (1992). The volume of serum producing 50 % hemolysis (ACH50) was determined, and the number of ACH50 U ml−1 was calculated for each group.
Respiratory burst activity (RBA)
The RBA of phagocytes was measured using the nitroblue tetrazolium (NBT, Sigma-Aldrich) assay following the method of Secombes (1990) with previously described modifications (Geng et al. 2012). Color development was measured at 630 nm with a spectrophotometer. KOH/DMSO was used as a blank.
Superoxide dismutase (SOD) and malondialdehyde (MDA) assay
SOD activity was determined with an enzymatic assay method using a reagent kit (Randox, Crumlin, UK), as described in Sun et al. (2010). MDA content was measured using barbituric acid reaction chronometry (Drape et al. 1993).
Phagocytic activity (PA)
PA of head kidney macrophages was determined following the method of Geng et al. (2012). The number of phagocytic cells per 100 adhered cells was microscopically determined. PA was calculated using the formula:
Total myeloperoxidase (MPO) activity
Total MPO activity in peripheral blood leukocytes was measured using the method described by Zhang et al. (2014a).
Immunoglobulin M (IgM) activity
Plasma total IgM levels were measured following the method described by Sharma et al. (2010). Total immunoglobulin was expressed as U mg−1.
Blood biochemical parameters assay
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were estimated using commercial kits (Shahsavani et al. 2010).
Challenge test
The 7-day lethal dose 50 (LD50) for A. hydrophila MTCC-1739 was 107 cfu ml−1 as determined earlier in our laboratory (Giri et al. 2012). Challenge tests were conducted at 15, 30, 45, and 60 days post-feeding. For each test, four fish from each tank (i.e., 4 fish × 3 tanks = 12 fish per group) were collected and injected intraperitoneally with 100 µl of phosphate-buffered saline (PBS) containing 1 × 107 live A. hydrophila. Another group of 12 fish (fed the basal diet during the feeding trial) were injected with 100 µl PBS and considered as negative control. The challenged fish were kept under observation for 15 days and fed basal diet. The mortality of fish in each tank was observed over the course of 15 days.
Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze the data. Multiple comparisons were performed with Tukey’s test to analyze the differences between treatments. All statistical analyses were performed using the OriginPro software (version 8; OriginLab Corporation, Northampton, U.S.A). The level of significance was set at p < 0.05, and the results are expressed as mean (S.E.M).
Results
Growth parameters
The results of the growth parameters (n = 15) study revealed that fish fed E2 diet for 30–60 days exhibited significantly higher WG than the control. The highest and second-highest WGs were recorded in fish that were fed E2 diet for 45 and 30 days, respectively (p < 0.05) (data not shown). Fish fed E3 diet had significantly higher WG only when fed for 30 days. The FCR was lower in the E2- and E3-fed groups, but the variation was not significant.
Immunological parameters
Fish that were orally supplemented with dietary emodin showed differences (p < 0.05) in specific and non-specific immune responses. A significantly higher LA was observed only in the E2- and E3-fed groups after 30 and 45 days of feeding (Table 1). The highest LA was observed in the E2-fed group after 45 days of feeding. The ACP activities in the emodin-fed groups were significantly higher after 30 and 45 days of feeding as compared to the control (Table 1). The maximum ACP activity was observed in the E2-fed group after 45 days of feeding. The RBA in the E2- and E3-fed groups was significantly higher than the control group after 30 and 45 days of feeding (Fig. 1a). After 45 days, emodin had no significant effect on RBA in fish. The PA (%) was found to be significantly higher in the E2- and E3-fed groups compared to the control during the entire trial period, and the highest PA was observed in the E2-fed group after 45 days of feeding (Fig. 1b). The MPO activity was significantly higher in the E2- and E3-fed groups after 15 and 45 days of feeding, compared to the control (Fig. 1c). The highest MPO activity was measured in fish fed E3 diet for 45 days. Regarding the plasma IgM level, a significant increase (p < 0.05) was observed in the emodin-fed groups after 30 and 45 days of feeding (Table 2), and the highest level was in the E2-fed group after 30 days of feeding. The lowest IgM level was observed in the E3-fed group at the end of the trial.
Antioxidant parameters
The SOD activity was significantly higher in the E2-fed group after 30, 45, and 60 days of feeding than in the control (Fig. 2a). The highest SOD activity was observed in the E2-fed group after 45 days of feeding. Significantly lower MDA activities were observed in the E2- and E3-fed groups after 30 and 45 days of feeding (Fig. 2b). Emodin feeding had no significant effect on MDA activities at 15 and 60 days of feeding (Fig. 2b).
Blood biochemical parameters
The plasma AST and ALT activities in emodin-fed groups were lower than in the control group at all sample points (Table 3). Significantly lower AST and ALT activity was recorded in the E2- and E3-fed groups after 30 and 45 days of feeding, with the lowest activities recorded in the E2-fed group.
Challenge test
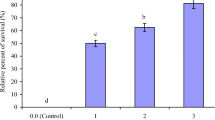
The results of the challenge test (n = 12 for each dietary group) are shown in Fig. 3. The challenge test revealed that different feeding patterns of emodin significantly affected A. hydrophila resistance in L. rohita. No mortality was recorded within 48 h post-challenge. The highest survival percentages were recorded in the fish groups that were fed E2 diet for 45 days (83.3 %), 30 days (75 %), and 60 days (58.3 %).
Discussion
Enhancing immune responses seems to be the most promising method of preventing diseases in fish. Application of herbal immunostimulants in aquaculture has received increasing attention over the past two decades. In the present study, significantly higher WG was observed in the E2-fed group after 30–60 days of feeding. In a similar study, emodin supplementation at 30 mg kg−1 for 4 weeks significantly enhanced the WG of M. amblycephala (Zhang et al. 2014a). The reduction in FCR value in treatment groups revealed that fish consumed dietary nutrients more efficiently when diet was supplemented with emodin. In line with our findings, several studies have shown that acute feeding of herbal extracts can increase the growth rate of fish when compared to long-term feeding (Xie et al. 2008; Geng et al. 2012; Zhang et al. 2014a). Long-term oral administration of immunostimulants may lead to immunosuppression (de Baulny et al. 1996).
As a first line of defense, various peptides such as lysozyme, antibodies, and complement factors inhibit the adhesion and colonization of microorganisms, leading to the prevention of infection and disease. Lysozyme disrupts the cell walls of certain pathogens and is a natural antagonist to harmful invaders such as parasites, bacteria, and viruses (Ellis 1999). In the present study, LA and ACP were significantly higher in fish groups fed with E2 or E3 diet for 30–45 days. Dietary emodin enhanced the serum LA in M. amblycephala (Ming et al. 2012). Dietary supplementation with 0.1 % anthraquinone extract from R. officinale for 10 weeks increased the LA in Macrobrachium rosenbergii (Liu et al. 2012). In line with the present study, Epinephelus bruneus fed for 30 days with 0.1–2 % S. japonica had enhanced (p < 0.05) complement activity and LA (Harikrishnan et al. 2011). Higher levels and longer periods of emodin supplementation had no significant effect on LA or ACP activity in the present study. This is likely because higher doses of immunostimulants for longer durations lead to immunosuppression (Zhang et al. 2014a).
Respiratory bursts, the increases in oxidation levels in phagocytes stimulated by foreign agents, are considered a vital indicator of nonspecific defense in fish (Jian and Wu 2003), where −O2 is the first product to be released (Harikrishnan et al. 2011). In the present study, enhanced RBA (p < 0.05) was observed in E2- and E3-fed groups after 30 and 45 days of feeding, and significantly higher PA was observed in E2- and E3-fed groups during the entire study period. MPO, which is abundantly stored and expressed in primary azurophilic granules of neutrophils, utilizes hydrogen peroxide during respiratory bursts to produce hypochlorous acid (Dalmo et al. 1997). MPO level was significantly higher in the emodin-fed groups after 30–45 days of trial. Oral administration of emodin at 30 mg kg−1 for 4 weeks significantly enhanced the RBA and MPO in M. amblycephala (Zhang et al. 2014a). In this study, the highest RBA, PA and MPO activities were observed in the E2-fed group after 30 and 45 days of feeding. These results indicate that a significant improvement in nonspecific immune responses occurs after 30–45 days of feeding with E2 diet. It is worth mentioning that the duration of nonspecific immune responses is always shorter than the specific immune responses in fish (Anderson 1992).
In vertebrates, the phagocytic process is followed by the production of highly microbicidal reactive oxygen molecules, such as superoxide anion (−O2), hydrogen peroxides (H2O2), and hydroxyl radical (OH−) (Di Giulo et al. 1993). MDA, the main component of lipid peroxides, has a strong biotoxicity and can damage cell structure and function (Freeman and Crapo 1982). SOD catalyzes the dismutation of the highly reactive (−O2) to less reactive H2O2 and functions in the main antioxidant defense pathway in response to oxidative stress (Fridovich 1995). The present study reveals that SOD production was significantly higher in fish fed E2 diet for 30–60 days. However, MDA content was significantly lower in E2- and E3-fed groups after 30–45 days of trial. Consistent with our study, dietary supplementation with emodin at 30 mg kg−1 for 4 weeks significantly enhanced the SOD content and reduced the MDA level in M. amblycephala (Zhang et al. 2014a). Ming et al. (2012) also noted similar results in M. amblycephala fed emodin and vitamin C.
The predominant antibody type in fish is high molecular weight immunoglobulin (Ig), often referred to as IgM. IgM is used to identify and neutralize foreign objects such as bacteria and viruses (Fridovich 1995). The plasma IgM level in treatment groups showed an increasing trend (p < 0.05) after 30–45 days of feeding and thereafter gradually decreased. Similar observations were made by several researchers (Giri et al. 2013; Sharma et al. 2010). In line with earlier reports, it can be suggested that stimulation of IgM level is a short-term phenomenon attributable to immunostimulants.
In this investigation, significantly lower AST and ALT levels were observed in E2- and E3-fed groups after 30 and 45 days of feeding. Previous studies have revealed that AST and ALT are the most important aminotransferases in fish and are commonly regarded as indicators of liver damage (Sheikhzadeh et al. 2012). Hence, this feeding pattern (i.e., 30–40 mg kg−1 emodin for 30–45 days) may reduce liver damage in fish. Similar trends in levels of AST and ALT were observed in grass carp (Ctenopharyngodon idella) (Lin et al. 1990) and Wuchang bream (Zhang et al. 2014a) under the similar feeding patterns.
Bacterial challenge tests provide opportunities to evaluate the effectiveness of immunostimulants in protecting against pathogens (Giri et al. 2013). We found that fish fed E2 diet for 45 days exhibited the highest survival rate (83.3 %), followed by fish fed E2 diet 30 days (75 %) and 60 days (58.3 %). Previous studies have revealed that dietary supplementation of 0.1 % anthraquinone extract for 10 weeks enhances the resistance of M. amblycephala against A. hydrophila (Liu et al. 2012). Feeding of 30 mg kg−1 emodin for 4 weeks enhanced the resistance of M. amblycephala against A. hydrophila challenge (Zhang et al. 2014a). Dietary feeding of W. somnifera root powder enhanced the resistance of L. rohita fingerlings against A. hydrophila (Sharma et al. 2010). The enhanced immune parameters such as LA, ACP, RBA, PA, MPO and IgM, and declines in biochemical parameters MDA, AST and ALT in the fish fed 30 mg kg−1 for 30–45 days might be associated with the improved resistance of fish against A. hydrophila and the resulting higher post-challenge survival percentages.
Conclusion
The present investigation reveals that supplementation of 30 mg kg−1 emodin for 30–45 days was associated with increased weight gain, LA, ACP, SOD, PA, RBA, MPO activity, and IgM level in L. rohita. Further, a decline in FCR, MDA, ALP, and AST content was noted in E2 fed group after 30–45 days of feeding. Taken together, these results suggest that 30 mg kg−1 emodin supplementation for 30–45 days is optimum to increase the growth performances, immunity, and disease resistance against pathogenic bacterial infection in L. rohita. This natural immunostimulant may be an alternative to prophylactic use of chemicals in freshwater aquaculture practice. However, effect of emodin through various modes of administration should be further investigated in order to explore its molecular mechanisms.
Abbreviations
- ACP:
-
Alternative complement pathway
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- FCR:
-
Feed conversion ratio
- FI:
-
Feed intake
- HASMC:
-
Human aortic smooth-muscle cell
- IgM:
-
Immunoglobulin M
- LA:
-
Lysozyme activity
- LD50 :
-
Lethal dose 50 %
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- PA:
-
Phagocytic activity
- PBS:
-
Phosphate buffer saline
- RBA:
-
Respiratory burst activity
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
- WG:
-
Weight gain
References
Anderson DP (1992) Immunostimulants, adjuvants and vaccine carriers in fish: application to aquaculture. Annu Rev Fish Dis 2:281–307
AOAC (1997) Official methods of analyses, 16th edn. Association of Official Analytical Chemists, Washington, DC
APHA, AWWA, WEF (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, American Water Works Association, Water Environment Association, Washington DC, pp 413–426
Austin B, Austin DA (2007) Bacterial fish pathogens: diseases of farmed and wild fish, 4th edn. Springer, Chichester
Bhadauria M (2010) Dose-dependent hepatoprotective effect of emodin against acetaminophen-induced acute damage in rats. Exp Toxicol Pathol 62:627–635
Chang CH, Lin CC, Yang JJ, Namba T, Hattori M (1996) Anti-inflammatory effects of emodin from ventilago leiocarpa. Am J Chin Med 24:139–142
Dalmo RA, Ingebrightsen K, Bogwald J (1997) Non-specific defense mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J Fish Dis 20:241–273
de Baulny MO, Quentel C, Fournier V, Lamour F, Le Gouvello R (1996) Effect of long term oral administration of beta-glucan as an immunostimulant or an adjuvant on some non-specific parameters of the immune response of turbot Scophthalmus maximus. Dis Aquat Org 26:139–147
Di Giulio RT, Habig C, Gallagher EP (1993) Effects of black rock harbor sediments on indices of biotransformation, oxidative stress, and DNA integrity in channel catfish. Aquat Toxicol 26:1–22
Drape HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M (1993) A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med 15:353–363
Ellis AE (1999) Immunity to bacteria in fish. Fish Shellfish Immunol 9:291–308
FAO (2012) The state of world fisheries and aquaculture, Rome. ISBN: 978-92-5-107225-7
Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47:412–426
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Geng X, Dong X-H, Tan B-P, Yang Q-H, Chi S-Y, Liu H-Y, Liu X-Q (2012) Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquac Nutr 18:46–55
Giri SS, Sen SS, Sukumaran V (2012) Effects of dietary supplementation of potential probiotic Pseudomonas aeuginosa VSG2 on the innate immunity and disease resistance of tropical freshwater fish Labeo rohita. Fish Shellfish Immunol 32:1135–1140
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34:660–666
Harikrishnan R, Kim J-S, Kim M-C, Balasundaram C, Heo M-S (2011) Styrax japonica supplementation diet enhances the innate immune response in Epinephelus bruneus against bacterial and protozoan infections. Exp Parasitol 129:260–265
Heo SK, Yun HJ, Park WH, Park SD (2008) Emodin inhibits TNF-alpha-induced human aortic smooth muscle cell proliferation via caspase and mitochondrial-dependent apoptosis. J Cell Biochem 105:70–80
Jian J, Wu Z (2003) Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218:1–9
Kuo YC, Tsai WJ, Meng HC, Chen WP, Yang LY, Lin CY (2001) Immune responses in human mesangial cells regulated by emodin from Polygonum hypoleucum Ohwi. Life Sci 68:1271–1286
Lin D, Mao YQ, Cai FS (1990) Nutritional lipid liver disease of grass carp (Ctenopharyngodon idella). Chin J Oceanol Limnol 4:363–373
Liu Bo, Ge X, Xie J, Xu P, He Y, Cui Y, Ming J, Zhou Q, Pan L (2012) Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol 32:1–7
Ming JH, Xie J, Xu P, Ge XP, Liu WB, Ye JY (2012) Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol 32:651–661
Rao VY, Das BK, Jyotyrmayee P, Chakrabarti R (2006) Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol 20:263–273
Sahu S, Das BK, Mishra BK, Pradhan J, Sarangi N (2006) Effect of Allium satium on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J Appl Ichthyol 22:1–6
Secombes CJ (1990) Isolation of salmonid macrophages and analysis of their killing activity. In: Stolen JS, Fletcher TC, Anderson DP, Robertson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS publications, New Jersey, pp 137–154
Shahsavani D, Mohri M, Gholipour Kanani H (2010) Determination of normal values of some blood serum enzymes in Acipenser stellatus Pallas. Fish Physiol Biochem 36:39–43
Sharma A, Deo AD, Riteshkumar ST, Chanu TI, Das A (2010) Effect of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish Shellfish Immunol 29:508–512
Sheikhzadeh N, Tayefi-Nasrabadi H, Oushani AK, Enferadi MHN (2012) Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 38:413–419
Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: does it really protect against infection. Fish Shellfish Immunol 15:71–90
Sun Y-Z, Yang H-L, Ma R-L, Lin W-Y (2010) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol 29:803–809
Xie J, Liu Bo, Zhou Q, Su Y, He Y, Pan L, Ge X, Xu P (2008) Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture 281:5–11
Yano T (1992) Assays of hemolytic complement activity. In: Stolen JS, Fletcher TC, Anderson DP, Kaattari SL, Rowley AF (eds) Techniques in fish immunology. SOS Publications, New Jersey, pp 131–141
Yin G, Ardo L, Thompson KD, Adams A, Jeney Z, Jeney G (2009) Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol 26:140–145
Zhang Y-Y, Liu Bo, Ge X-P, Liu W-B, Xie J, Ren M, Cui Y-T, Xia S-l, Chen R, Zhou Q, Pan L, Yu Y (2014a) The influence of various feeding patterns of emodin on growth, non-specific immune responses, and disease resistance to Aeromonas hydrophila juvenile Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol 36(1):187–193
Zhang Y-Y, Liu B, Ge X-P, Liu W-B, Xie J, Ren M, Chen R, Zhou Q, Pan L (2014b) Comparative study of antibacterial properties of emodin and enrofloxacin against Aeromonas hydrophila. Isr J Aquac Bamidgeh 66. doi:IJA_66.2014.976
Acknowledgments
First author is a BK21 PLUS post-doctoral fellow at Seoul National University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Giri, S.S., Jai Suda, S., Sukumaran, V. et al. Dietary emodin affects the growth performance, immune responses, and disease resistance of Labeo rohita against Aeromonas hydrophila . Aquacult Int 24, 85–99 (2016). https://doi.org/10.1007/s10499-015-9910-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-015-9910-y