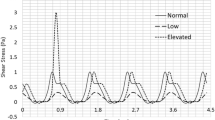
Abstract
To better understand the mechanisms leading to the formation of thrombi of hazardous sizes in the bulk of the blood, we have developed a kinetic model of shear-induced platelet aggregation (SIPA). In our model, shear rate regulates a mass-conservative population balance equation which computes the aggregation and disaggregation of platelets in a cluster mass distribution. Aggregation is modeled by the Smoluchowski coagulation equation, and disaggregation is incorporated using the aggregate breakup model of Pandya and Spielman. Previous experimental data for SIPA have been correlated with a special case of this model where only the two-body collision of free platelets was considered. However, the two-body collision theory is oblivious to the steady-state condition, and it required the use of a shear-dependent aggregation efficiency parameter to fit it to experimental data. Our method not only predicts steady states but also correlates with literature data without employing a shear-dependent aggregation efficiency.








Similar content being viewed by others
References
Bäbler, M. U., M. Morbidelli. Analysis of the aggregation-fragmentation population balance equation with application to coagulation. J. Colloid Interface Sci. 316(2):428–441, 2007. doi:10.1016/j.jcis.2007.08.029.
Barthelmes, G., S. Pratsinis, H. Buggisch. Particle size distributions and viscosity of suspensions undergoing shear-induced coagulation and fragmentation. Chem. Eng. Sci. 58(13): 2893–2902, 2003. doi:10.1016/S0009-2509(03)00133-7.
Bell, D. N., S. Spain, H. L. Goldsmith. Adenosine diphosphate-induced aggregation of human platelets in flow through tubes. II. Effect of shear rate, donor sex, and ADP concentration. Biophys. J. 56(5):829–843, 1989. Doi:10.1016/S0006-3495(89)82729-8.
Blatz, P. J., A. V. Tobolsky. Note on the kinetics of systems manifesting simultaneous polymerization-depolymerization phenomena. J. Phys. Chem. 49(2):77–80, 1945. doi:10.1021/j150440a004.
Born, G. V. R., M. Hume. Effects of the numbers and sizes of platelet aggregates on the optical density of plasma. Nature 215(5105):1027–1029, 1967. doi:10.1038/2151027a0.
Chang, H. N., C. R. Robertson. Platelet aggregation by laminar shear and Brownian motion. Ann. Biomed. Eng. 4(2):151–183, 1976. doi:10.1007/BF02363645
David, P., A. C. Nair, V. Menon, D. Tripathi. Laser light scattering studies from blood platelets and their aggregates. Colloids Surf. B 6(2):101–114, 1996. doi:10.1016/0927-7765(95)01236-2.
Diamond, S. L. Systems biology of coagulation. J. Thromb. Haemostasis 11(Suppl.1):224–232, 2013. doi:10.1111/jth.12220
Frojmovic, M. M., J. G. Milton. Human platelet size, shape, and related functions in health and disease. Physiol. Rev. 62(1):185–261, 1982.
Goldsmith, H. L., D. N. Bell, S. Braovac, A. Steinberg, F. McIntosh. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys. J. 69(4):1584–95, 1995. Doi:10.1016/S0006-3495(95)80031-7.
Goldsmith, H. L., D. N. Bell, S. Spain, F. A. McIntosh. Effect of red blood cells and their aggregates on platelets and white cells in flowing blood. Biorheology 36(5–6):461–468, 1999.
Goldsmith, H. L., S. Spain. Margination of leukocytes in blood flow through small tubes. Microvasc. Res. 27(2):204–222, 1984. doi:10.1016/0026-2862(84)90054-2.
Huang, P. Y., J. D. Hellums. Aggregation and disaggregation kinetics of human blood platelets: Part III. The disaggregation under shear stress of platelet aggregates. Biophys. J. 65(1):354–361 (1993a). doi:10.1016/S0006-3495(93)81080-4.
Huang, P. Y., J. D. Hellums. Aggregation and disaggregation kinetics of human blood platelets: Part I. Development and validation of a population balance method. Biophys. J. 65(1):334–43, 1993c. doi:10.1016/S0006-3495(93)81078-6.
Huang, P. Y., J. D. Hellums. Aggregation and disaggregation kinetics of human blood platelets: Part II. Shear-induced platelet aggregation. Biophys. J. 65(1):344–53, 1993b. doi:10.1016/S0006-3495(93)81079-8.
Hund, S. J., J. F. Antaki, An extended convection diffusion model for red blood cell-enhanced transport of thrombocytes and leukocytes. Phys. Med. Biol. 54(20):6415–6435, 2009. doi:10.1088/0031-9155/54/20/024.
Kao, S. V., S. G. Mason. Dispersion of particles by shear. Nature 253(5493):619–621, 1975. doi:10.1038/253619a0.
Kostoglou, M., A. J. Karabelas. On the self-similarity of the aggregationGçôfragmentation equilibrium particle size distribution. J. Aerosol Sci. 30(2):157–162, 1999. doi:10.1016/S0021-8502(98)00045-7.
Kramer, T., Clark, M. Incorporation of aggregate breakup in the simulation of orthokinetic coagulation. J. Colloid Interface Sci. 216(1):116–126 (1999). doi:10.1006/jcis.1999.6305.
Kroll, M. H., J. D. Hellums, L. V. McIntire, A. I. Schafer, J. L. Moake. Platelets and shear stress. Blood 88(5):1525–1541, 1996.
Kuharsky, A. L., A. L. Fogelson. Surface-mediated control of blood coagulation: the role of binding site densities and platelet deposition. Biophys. J. 80(3):1050–1074, 2001. doi:10.1016/S0006-3495(01)76085-7.
Kumar, S., Ramkrishna, D. On the solution of population balance equations by discretizationGçöI. A fixed pivot technique. Chem. Eng. Sci. 51(8):1311–1332, 1996. doi:10.1016/0009-2509(96)88489-2.
Landolfi, R., R. De Cristofaro, E. De Candia, B. Rocca, B. Bizzi. Effect of fibrinogen concentration on the velocity of platelet aggregation. Blood 78(2):377–381, 1991. doi:10.1016/0049-3848(91)90490-N.
Lattuada, M., P. Sandkühler, H. Wu, J. Sefcik, M. Morbidelli. Aggregation kinetics of polymer colloids in reaction limited regime: experiments and simulations. Adv. Colloid Interface Sci. 103(1):33–56, 2003. doi:10.1016/S0001-8686(02)00082-9.
Leiderman, K., A. L. Fogelson. Grow with the flow: a spatial-temporal model of platelet deposition and blood coagulation under flow. Math. Med. Biol. 28(1):47–84, 2011. doi:10.1093/imammb/dqq005.
Lin, M. Y., H. M. Lindsay, D. A. Weitz, R. C. Ball, R. Klein, P. Meakin. Universality in colloid aggregation. Nature 339(6223):360–362, 1989. doi:10.1038/339360a0.
Moiseyev, G., P. Z. Bar-Yoseph. Computational modeling of thrombosis as a tool in the design and optimization of vascular implants. J. Biomech. 46(2):248–252 (2013). doi:10.1016/j.jbiomech.2012.11.002.
Nesbitt, W. S., E. Westein, F. J. Tovar-Lopez, E. Tolouei, A. Mitchell, J. Fu, J. Carberry, A. Fouras, S. P. Jackson. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15(6):665–673, 2009. doi:10.1038/nm.1955.
Oliphant, T. E. Python for scientific computing. Comput. Sci. Eng. 9(3):10–20, 2007. doi:10.1109/MCSE.2007.58.
Pandya, J., L. Spielman. Floc breakage in agitated suspensions: theory and data processing strategy. J. Colloid Interface Sci. 90(2):517–531, 1982. doi:10.1016/0021-9797(82)90317-4.
Pandya, J., L. Spielman. Floc breakage in agitated suspensions: effect of agitation rate. Chem. Eng. Sci. 38(12):1983–1992, 1983. doi:10.1016/0009-2509(83)80102-X.
Paulus, J. M. Platelet size in man. Blood 46(3):321–36, 1975.
Pedocchi, F., I. Piedra-Cueva. Camp and SteinGçös velocity gradient formalization. J. Environ. Eng. 131(10):1369–1376, 2005. doi:10.1061/(ASCE)0733-9372(2005)131:10(1369).
Reif, F. Statistical Physics 398 (McGraw-Hill, New York, 1967).
Saffman, P. G., J. S. Turner. On the collision of drops in turbulent clouds. J. Fluid Mech. 1(01):16–30, 1956. doi:10.1017/S0022112056000020.
Schneider, S. W., S. Nuschele, A. Wixforth, C. Gorzelanny, A. Alexander-Katz, R.R. Netz, M.F. Schneider. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA. 104(19):7899–903,2007. doi:10.1073/pnas.0608422104.
Singh, I., E. Themistou, L. Porcar, S. Neelamegham. Fluid shear induces conformation change in human blood protein von Willebrand factor in solution. Biophys. J. 96(6):2313–2320, 2009. doi:10.1016/j.bpj.2008.12.3900.
Slomkowski, S, J. V. Alemán, R. G. Gilbert, M. Hess, K. Horie, R. G. Jones, P. Kubisa, I. Meisel, W. Mormann, S. Penczek, R. F. T. Stepto. Terminology of polymers and polymerization processes in dispersed systems (IUPAC Recommendations 2011). Pure Appl. Chem. 83(12):2229–2259, 2011. doi:.10.1351/PAC-REC-10-06-03
Soos, M, J. Sefcik, M. Morbidelli. Investigation of aggregation, breakage and restructuring kinetics of colloidal dispersions in turbulent flows by population balance modeling and static light scattering. Chem. Eng. Sci. 61(8):2349–2363, 2006. doi:10.1016/j.ces.2005.11.001.
Sorensen, C. M. The mobility of fractal aggregates: a review. Aerosol Sci. Technol. 45(7):765–779, 2011. doi:10.1080/02786826.2011.560909.
Sorensen, E. N., G. W. Burgreen, W. R. Wagner, J. F. Antaki, Computational simulation of platelet deposition and activation: II. Results for Poiseuille flow over collagen. Ann. Biomed. Eng. 27(4):449–458, 1999.
Sorensen, E. N., G. W. Burgreen, W. R. Wagner, J. F. Antaki. Computational simulation of platelet deposition and activation: I. Model development and properties. Ann. Biomed. Eng. 27(4):436–48, 1999.
Spicer, P. T., S. E. Pratsinis. Coagulation and fragmentation: universal steady-state particle-size distribution. AIChE J. 42(6):1612–1620, 1996. doi:10.1002/aic.690420612.
Spicer, P. T., S. E. Pratsinis, J. Raper, R. Amal, G. Bushell, G. Meesters. Effect of shear schedule on particle size, density, and structure during flocculation in stirred tanks. Powder Technol. 97(1):26–34, 1998. doi:10.1016/S0032-5910(97)03389-5.
Spicer, P. T., S. E. Pratsinis, M. D. Trennepohl, G. H. M. Meesters. Coagulation and fragmentation: the variation of shear rate and the time lag for attainment of steady state. Ind. Eng. Chem. Res. 35(9):3074–3080, 1996. doi:10.1021/ie950786n.
von Smoluchowski, M. R. Drei Vorträge über Diffusion, Brownsche Molekularbewegung und Koagulation von Kolloidteilchen. Physikalische Zeitschrift 17:585–599, 1916.
von Smoluchowski, M. R., Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Zeitschrift fuer physikalische Chemie 92(1912):129–168, 1917.
Westein, E., A. D. van der Meer, M. J. E. Kuijpers, J. P. Frimat, A. van den Berg, J. W. M. Heemskerk. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl. Acad. Sci. USA 110(4):1357–1362, 2013. doi:10.1073/pnas.1209905110.
Xia, Z., M. M. Frojmovic. Aggregation efficiency of activated normal or fixed platelets in a simple shear field: effect of shear and fibrinogen occupancy. Biophys. J. 66(6):2190–201, 1994. doi:10.1016/S0006-3495(94)81015-X.
Acknowledgments
This work is supported by the Higher Education Authority of Ireland under the Programme for Research in Third Level Institutions through the Structured Ph.D. in Biomedical Engineering and Regenerative Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hellmuth, R., Bruzzi, M.S. & Quinlan, N.J. Analysis of Shear-Induced Platelet Aggregation and Breakup. Ann Biomed Eng 44, 914–928 (2016). https://doi.org/10.1007/s10439-015-1409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1409-1