Abstract
Calcium is the primary signalling component of excitation-contraction coupling, the process linking electrical excitability of cardiac muscle cells to coordinated contraction of the heart. Understanding \({\text{Ca}}^{2+}\) handling processes at the cellular level and the role of intercellular communication in the emergence of multicellular synchronization are key aspects in the study of arrhythmias. To probe these mechanisms, we have simulated cellular interactions on large scale arrays that mimic cardiac tissue, and where individual cells are represented by a mathematical model of intracellular \({\text{Ca}}^{2+}\) dynamics. Theoretical predictions successfully reproduced experimental findings and provide novel insights on the action of two pharmacological agents (ionomycin and verapamil) that modulate \({\text{Ca}}^{2+}\) signalling pathways via distinct mechanisms. Computational results have demonstrated how transitions between local synchronisation events and large scale wave formation are affected by these agents. Entrainment phenomena are shown to be linked to both intracellular \({\text{Ca}}^{2+}\) and coupling-specific dynamics in a synergistic manner. The intrinsic variability of the cellular matrix is also shown to affect emergent patterns of rhythmicity, providing insights into the origins of arrhythmogenic \({\text{Ca}}^{2+}\) perturbations in cardiac tissue in situ.







Similar content being viewed by others
References
Bers, D. M. Cardiac excitation-contraction coupling. Nature 415:198–205, 2002.
Christ, G. J., D. C. Spray, M. El-Sabban, L. K. Moore, and P. R. Brink. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ. Res. 79:631–646, 1996.
Claycomb, W. C., N. A. Lanson, B. S. Stallworth, D. B. Egeland, J. B. Delcarpio, A. Bahinski, and N. J. Izzo Jr. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl Acad. Sci. U.S.A. 95:2979–2984, 1998.
Dehmelt, L., and P. I. H. Bastiaens. Spatial organization of intracellular communication: insights from imaging. Nat. Rev. Mol. Cell. Biol. 11:440–452, 2010.
Dhillon, P. S., R. Gray, P. Kojodjojo, R. Jabr, R. Chowdhury, C. H. Fry, and N. S. Peters. Relationship between gap-junctional conductance and conduction velocity in mammalian myocardium. Circ. Arrhythm. Electrophysiol. 6:1208–1214, 2013.
Drikakis, D., J. Lechuga, and S. Pal. Effects of shock waves on biological membranes: a molecular dynamics study. J. Comput. Theor. Nanos. 6:1437–1442, 2009.
Falcke, M. Reading the patterns in living cells—the physics of Ca2+ signaling. Adv. Phys. 53:255–440, 2004.
George, C. H., G. V. Higgs, and F. A. Lai. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ. Res. 93:531–540, 2003.
George, C. H., D. Parthimos, and N. C. Silvester. A network-oriented perspective on cardiac calcium signaling. Am. J. Physiol. Cell Physiol. 303:C897–C910, 2012.
Glass L. Synchronization and rhythmic processes in physiology. Nature 410:277–284, 2001.
Guevara M. R., L. Glass, and A. Shrier. Phase locking, period doubling bifurcations and irregular dynamics in periodically stimulated cardiac cells. Science 214:1350–1353, 1981.
Jacobsen, J. C., C. Aalkjaer, V. V. Matchkov, H. Nilsson, J. J. Freiberg, and N. H. Holstein-Rathlou. Heterogeneity and weak coupling may explain the synchronization characteristics of cells in the arterial wall. Philos. Trans. A. Math. Phys. Eng. Sci. 366:3483–3502, 2008.
Jordan, J. D., E. M. Landau, and R. Iyengar. Signaling networks: the origins of cellular multitasking. Cell 103:193–200, 2000.
Ter Keurs, H. E. D. J., and P. A. Boyden. Calcium and arrhythmogenesis. Physiol. Rev. 87:457–506, 2007.
Kholodenko, B., M. B. Yaffe, and W. Kolch. Computational approaches for analyzing information flow in biological networks. Sci. Signal. 5:re1, 2012.
Kim, J.-R., D. Shin, S. H. Jung, P. Heslop-Harrison, and K.-H. Cho. A design principle underlying the synchronization of oscillations in cellular systems. J. Cell Sci. 123:537–543, 2010.
Kitano, H. Grand challenges in systems physiology. Front. Physiol. 1:3, 2010.
Koenigsberger, M., R. Sauser, and J.-J. Meister. Emergent properties of electrically coupled smooth muscle cells. Bull. Math. Biol. 67:1253–1272, 2005.
Krikler, D. M. Verapamil in arrhythmia. Br. J. Clin. Pharmacol. 21(Suppl 2):1835:1895, 1986.
Kurz, F. T., M. A. Aon, B. O’Rourke, and A. A. Armoundas. Spatio-temporal oscillations of individual mitochondria in cardiac mycocytes reveal modulation of synchronized mitochondrial clusters. Proc. Natl Acad. Sci. U.S.A. 107:14315–14320, 2010.
Lakatta, E. G., V. A. Maltsev, K. Y. Bogdanov, M. D. Stern, and T. M. Vinogradova. Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ. Res. 92:E45–E50, 2003.
Lee, Y.-S., O. Z. Liu, and E. A. Sobie. Decoding myocardial Ca2+ signals across multiple spatial scales: a role for sensitivity analysis. J. Mol. Cell. Cardiol. 58:92–99, 2003.
Li, X., and J. M. Simard. Multiple connexins form gap junction channels in rat basilar artery smooth muscle cells. Circ. Res. 84:1277–1284, 1999.
Miura, M., P. A. Boyden, and H. E. ter Keurs. Calcium waves during triggered propagated contractions in intact trabeculae. Am. J. Physiol. 274:H266–H276, 1998.
Moreno, A. P., M. B. Rook, G. L. Fishman, and D. C. Spray. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys. J. 67:113–119, 1994.
Nakamura, N., T. Yamazawa, Y. Okubo, and M. Iino. Temporal switching and cell-to-cell variability in Ca2+ release activity in mammalian cells. Mol. Syst. Biol. 5:247, 2009.
Nivala, M., C. Y. Ko, M. Nivala, J. M. Weiss, and Z. Qu. Criticality in intracellular calcium signaling in cardiac myocytes. Biophys. J. 102:2433–2442, 2012.
Novak, B., and J. J. Tyson. Design principles of biochemical oscillators. Nat. Rev. Mol. Cell Biol. 9:981–991, 2008.
Parthimos, D., D. H. Edwards, and T. M. Griffith. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am. J. Physiol. Heart Circ. Physiol. 46:H1119–H1144, 1999.
Parthimos, D., R. E. Haddock, C. E. Hill, and T. Griffith. Dynamics of a three-variable nonlinear model of vasomotion: comparison of theory and experiment. Biophys. J. 93:1534–1556, 2007.
Postma, A.V., I. Denjoy, T. M. Hoorntje, J. M. Lupoglazoff, A. Da Costa, P. Sebillon, M. M. A. M. Mannens, A. A. M. Wilde, and P. Guicheney. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 91:E21–E26, 2002.
Roell, W., T. Lewalter, P. Sasse, Y. N. Tallini, B. R. Choi, M. Breithart, R. Doran, U. M. Becher, S. M. Hwang, T. Bostani, J. von Maltzahn, A. Hofmann, S. Reining, B. Eiberger, B. Gabris, A. Pfeifer, A. Welz, K. Willecke, G. Salama, J. W. Schrickel, M. I. Kotlikoff, and B. K. Fleischmann. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450:819–826, 2007.
Rudy, Y. Conductive bridges in cardiac tissue. A beneficial role or an arrhythmogenic substrate? Circ. Res. 94:709–711, 2004.
Scoote, M., A. J. Williams. Myocardial calcium signalling and arrhythmia pathogenesis. Biochem. Biophys. Res. Commun. 322:1286–1309, 2004.
Severs, N. J., S. R. Coppen, E. Dupont, H. I. Yeh, Y. S. Ko, and T. Matsushita. Gap junction alterations in human cardiac disease. Cardiovasc. Res. 62:368–377, 2004.
Shaw, R. M., and Y. Rudy. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ. Res. 81:727–741, 1997.
Stern, M. D., L. A. Maltseva, M. Juhaszova, S. J. Sollott, E. G. Lakatta, and V. A. Maltsev. Hierarchical clustering of ryanodine receptors enables emergence of a calcium clock in sinoatrial node cells. J. Gen. Physiol. 143:577–604, 2014.
Tribulova, N., S. Seki, J. Radosinska, P. Kaplan, E. Babusikova, V. Knezl, and S. Mochizuki. Myocardial Ca2+ handling and cell-to-cell coupling, key factors in prevention of sudden cardiac death. Can. J. Physiol. Pharm. 87:1120–1129, 2009.
Tyson, J. J., K. C. Chen, and B. Novak. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15:221–231, 2003.
Weiss, J. N. How does falling out of phase of intracellular calcium and action potentials across the heart’s wall spell the beginning of chaos for the heart? Dialog. Cardiovasc. Med. 15:302–310, 2010.
Weiss, J. N., M. Nivala, A. Garfinkel, and Z. Qu. Alternans and arrrhythmias: from cell to heart. Circ. Res. 108:98–112, 2011.
Zhang, S., Z. Zhou, Q. Gong, J. C. Makielski, and C. T. January. Mechanisms of block and identification of the verapamil binding domain to HEGR potassium channels. Circ. Res. 84:989–998, 1999.
Acknowledgments
This work was partly supported by Grants from the British Heart Foundation (FS/09/028/27602, FS/06/082/21723), Heart Research UK (RG2559), Wellcome Trust (094219/Z/10/Z), and the Cardiff Partnership Fund. The authors also acknowledge the financial support provided by the Sêr Cymru National Research Network in Advanced Engineering and Materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Estefanía Peña oversaw the review of this article.
Appendix
Appendix
Mathematical Formulation
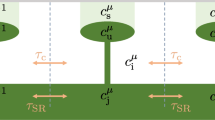
For each individual cell, the system is described by three variables: \(x\) = \([{\text{Ca}}^{2+}]_i\) represent the cytosolic free \({\text{Ca}}^{2+}\) concentration, \(y\) = \([{\text{Ca}}^{2+}]_{SR}\) the \({\text{Ca}}^{2+}\) concentration in the SR and \(z\) the cell membrane potential.
The electric reversal potentials with respect to \({\text{Ca}}^{2+}\) and \({\text{Na}}^{+}\) are determined from the Nernst equation, see Parthimos et al. for details.29,30 The different terms and associated fixed parameter values can be found in Table 1. The subscript \(r\) refers to RyR-mediated CICR, as opposed to InsP\(_3\)-induced \({\text{Ca}}^{2+}\) release (not included in the present formulation).
For each SMC \(i\), the set of its nearest neighbours \(j \in N_i\) consist of at most 6 neighbours, disposed at the vertices of an hexagon, depending on its position within the domain or at the boundary. Two terms:
are added to (A-1a) and (A-1c), respectively, to model \({\text{Ca}}^{2+}\) and electrical coupling.
Rights and permissions
About this article
Cite this article
Boileau, E., George, C.H., Parthimos, D. et al. Synergy Between Intercellular Communication and Intracellular Ca2+ Handling in Arrhythmogenesis. Ann Biomed Eng 43, 1614–1625 (2015). https://doi.org/10.1007/s10439-014-1243-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1243-x