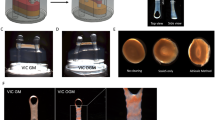
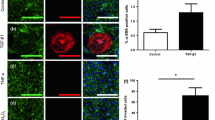
Abstract
Aortic valve interstitial cells (VIC) can exhibit phenotypic characteristics of fibroblasts, myofibroblasts, and smooth muscle cells. Others have proposed that valve cells become activated and exhibit myofibroblast or fibroblast characteristics during disease initiation and progression; however, the cues that modulate this phenotypic change remain unclear. We hypothesize that the mechanical forces experienced by the valve play a role in regulating the native phenotype of the valve and that altered mechanical forces result in an activated phenotype. Using a novel ex vivo cyclic stretch and pressure bioreactor, we subjected porcine aortic valve (AV) leaflets to combinations of normal and pathological stretch and pressure magnitudes. The myofibroblast markers α-SMA and Vimentin, along with the smooth muscle markers Calponin and Caldesmon, were analyzed using immunohistochemistry and immunoblotting. Tissue structure was analyzed using Movat’s pentachrome staining. We report that pathological stretch and pressure inhibited the contractile and possibly myofibroblast phenotypes as indicated by downregulation of the proteins α-SMA, Vimentin, and Calponin. In particular, Calponin downregulation implies depolymerization of actin filaments and possible conversion to a more synthetic (non-contractile) phenotype. This agreed well with the increase in spongiosa and fibrosa thickness observed under elevated pressure and stretch that are typically indicative of increased matrix synthesis. Our study therefore demonstrates how cyclic stretch and pressure may possibly act together to modulate the AVIC phenotype.








Similar content being viewed by others
References
Balachandran, K., et al. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann. Biomed. Eng. 34(11):1655–1665, 2006.
Balachandran, K., et al. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps—implications for degenerative aortic valve disease? Am. J. Physiol. Heart Circ. Physiol. 296(3):H756–H764, 2009.
Balachandran, K., et al. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am. J. Pathol. 177(1):49–57, 2010.
Chester, A. H., and P. M. Taylor. Molecular and functional characteristics of heart-valve interstitial cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362(1484):1437–1443, 2007.
Della Rocca, F., et al. Cell composition of the human pulmonary valve: a comparative study with the aortic valve-the VESALIO* project. Ann. Thorac. Surg. 70(5):1594–1600, 2000.
Desmouliere, A., et al. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int. J. Biochem. Cell Biol. 29(1):19–30, 1997.
El-Mezgueldi, M. Calponin. Int. J. Biochem. Cell Biol. 28(11):1185–1189, 1996.
Franke, W. W., et al. Intermediate sized filaments of human endothelial cells. J. Cell Biol. 81(3):570–580, 1979.
Galqzkiewicz, B., et al. Polymerization of G-actin by caldesmon. FEBS Lett. 184(1):144–149, 1985.
Hossain, M. M., et al. h2-Calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J. Biol. Chem. 280(51):42442–42453, 2005.
Ingber, D. Mechanobiology and diseases of mechanotransduction. Ann. Med. 35(8):564–577, 2003.
Jian, B., et al. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 75(2):457–465, 2003; (discussion 465–466).
Kake, T., et al. Calponin induces actin polymerization at low ionic strength and inhibits depolymerization of actin filaments. Biochem J 312:587–592, 1995.
Konduri, S., et al. Normal physiological conditions maintain the biological characteristics of porcine aortic heart valves: an ex vivo organ culture study. Ann. Biomed. Eng. 33(9):1158–1166, 2005.
Liu, A. C., V. R. Joag, and A. I. Gotlieb. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 171(5):1407–1418, 2007.
Marston, S. B., and C. S. Redwood. The molecular anatomy of caldesmon. Biochem. J. 279(1):1–16, 1991.
Mehta, D., and S. J. Gunst. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J. Physiol. Lond. 519(3):829–840, 1999.
Merryman, W. D., et al. Synergistic effects of cyclic tension and transforming growth factor-beta1 on the aortic valve myofibroblast. Cardiovasc. Pathol. 16(5):268–276, 2007.
Mohler, III, E. R. Mechanisms of aortic valve calcification. Am. J. Cardiol. 94(11):1396–1402, 2004.
North, A. J., et al. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J. Cell Sci. 107(3):437–444, 1994.
Philippe, S., et al. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J. Biomech. Eng. 130(3):035001, 2008.
Rabkin, S. W. The association of hypertension and aortic valve sclerosis. Blood Press. 14(5):264–272, 2005.
Rabkin, E., et al. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104(21):2525–2532, 2001.
Rabkin-Aikawa, E., et al. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 13(5):841–847, 2004.
Schneider, P. J., and J. D. Deck. Tissue and cell renewal in the natural aortic valve of rats: an autoradiographic study. Cardiovasc. Res. 15(4):181–189, 1981.
Schnittler, H. J., T. Schmandra, and D. Drenckhahn. Correlation of endothelial vimentin content with hemodynamic parameters. Histochem. Cell Biol. 110(2):161–167, 1998.
Sucosky, P., et al. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29(2):254–260, 2009.
Taylor, P. M., et al. The cardiac valve interstitial cell. Int. J. Biochem. Cell Biol. 35(2):113–118, 2003.
Wang, N., and D. Stamenovic. Mechanics of vimentin intermediate filaments. J. Muscle Res. Cell Motil. 23(5):535–540, 2002.
Xing, Y., et al. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann. Biomed. Eng. 32(4):555–562, 2004.
Xing, Y., et al. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann. Biomed. Eng. 32(11):1461–1470, 2004.
Acknowledgments
National Science Foundation through the Engineering Research Center program at Georgia Tech/Emory Center for the Engineering of Living Tissues under award EEC-9731643. Holifield Farms for providing porcine hearts for the research. Patrick Thayer was supported by the President’s Undergraduate Research Award (PURA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Thayer, P., Balachandran, K., Rathan, S. et al. The Effects of Combined Cyclic Stretch and Pressure on the Aortic Valve Interstitial Cell Phenotype. Ann Biomed Eng 39, 1654–1667 (2011). https://doi.org/10.1007/s10439-011-0273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0273-x