Abstract
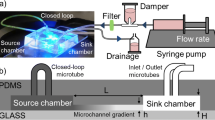
We report a microfluidic device that can rapidly and accurately generate various concentration gradients in a controllable manner for the chemotaxis study of motile bacterial cells by integrating hydrogel into polydimethylsiloxane (PDMS) microchannels. We performed numerical simulations for both the PDMS-sealed hydrogel hybrid device and a representative conventional hydrogel-based device to theoretically compare their characteristics. In addition, we experimentally demonstrated that the PDMS-sealed hydrogel device not only produces various linear and nonlinear concentration gradients without flow-induced shear stresses on motile bacterial cells but also exhibits remarkable advantages over conventional hydrogel-based devices. For example, the PDMS-sealed hydrogel device can be used for fast and accurate generation of various concentration gradients, prevents dehydration of hydrogel and evaporation of solutions, directs diffusion of chemicals such as chemoattractants, exhibits long-term durability, and is easy to handle. Because the hydrogel used is biocompatible and arbitrary concentration profiles can be easily designed and produced on a chip, we believe that not only the PDMS-sealed hydrogel fabrication method but also the versatile concentration gradient generation device can be used for various studies on interaction between chemicals and cells including bacterial chemotaxis assays.






Similar content being viewed by others
References
Adler J (1966) Chemotaxis in Bacteria. Science 153:708–716
Ahmed T, Shimizu TS, Stocker R (2010a) Bacterial chemotaxis in linear and nonlinear steady microfluidic gradients. Nano Lett 10:3379–3385
Ahmed T, Shimizu TS, Stocker R (2010b) Microfluidics for bacterial chemotaxis. Integr Biol 2:604–629
Cheng SY, Heilman S, Wasserman M, Archer S, Shuler ML, Wu MM (2007) A hydrogel-based microfluidic device for the studies of directed cell migration. Lab Chip 7:763–769
Choi E, Chang HK, Lim CY, Kim T, Park J (2012) Concentration gradient generation of multiple chemicals using spatially controlled self-assembly of particles in microchannels. Lab Chip 12:3968–3975
Chueh BH, Huh D, Kyrtsos CR, Houssin T, Futai N, Takayama S (2007) Leakage-free bonding of porous membranes into layered microfluidic array systems. Anal Chem 79:3504–3508
Diao JP, Young L, Kim S, Fogarty EA, Heilman SM, Zhou P, Shuler ML, Wu MM, Delisa MP (2006) A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab Chip 6:381–388
Ellegood J, Hanstock CC, Beaulieu C (2005) Trace apparent diffusion coefficients of metabolites in human brain using diffusion weighted magnetic resonance spectroscopy. Magn Reson Med 53:1025–1032
Englert DL, Manson MD, Jayaraman A (2010) Investigation of bacterial chemotaxis in flow-based microfluidic devices. Nat Protoc 5:864–872
Gorman BR, Wikswo JP (2008) Characterization of transport in microfluidic gradient generators. Microfluid Nanofluid 4:273–285
Haessler U, Kalinin Y, Swartz MA, Wu MW (2009) An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices 11:827–835
Herzmark P, Campbell K, Wang F, Wong K, El-Samad H, Groisman A, Bourne HR (2007) Bound attractant at the leading versus the trailing edge determines chemotactic prowess. Proc Natl Acad Sci USA 104:13349–13354
Hill J, Kalkanci O, Mcmurry JL, Koser H (2007) Hydrodynamic surface interactions enable Escherichia coli to seek efficient routes to swim upstream. Phys Rev Lett 98:068101
Jeon NL, Baskaran H, Dertinger SKW, Whitesides GM, Van de Water L, Toner M (2002) Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol 20:826–830
Kim M, Kim T (2010) Diffusion-based and long-range concentration gradients of multiple chemicals for bacterial chemotaxis assays. Anal Chem 82:9401–9409
Kim T, Pinelis M, Maharbiz MM (2009) Generating steep, shear-free gradients of small molecules for cell culture. Biomed Microdevices 11:65–73
Kim M, Kim SH, Lee SK, Kim T (2011) Microfluidic device for analyzing preferential chemotaxis and chemoreceptor sensitivity of bacterial cells toward carbon sources. Analyst 136:3238–3243
Kim M, Jia M, Kim T (2013) Ion concentration polarization in a single and open microchannel induced by a surface-patterned perm-selective film. Analyst 138:1370–1378
Lebrun L, Junter GA (1993) Diffusion of sucrose and dextran through agar-gel membranes. Enzyme Microbial Technol 15:1057–1062
Qin D, Xia YN, Whitesides GM (2010) Soft lithography for micro- and nanoscale patterning. Nat Protoc 5:491–502
Sahai R, Cecchini M, Klingauf M, Ferrari A, Martino C, Castrataro P, Lionetti V, Menciassi A, Beltram F (2011) Microfluidic chip for spatially and temporally controlled biochemical gradient generation in standard cell-culture Petri dishes. Microfluid Nanofluid 11:763–771
Si GW, Yang W, Bi SY, Luo CX, Ouyang Q (2012) A parallel diffusion-based microfluidic device for bacterial chemotaxis analysis. Lab Chip 12:1389–1394
Stock AM (1999) A nonlinear stimulus-response relation in bacterial chemotaxis. Proc Natl Acad Sci USA 96:10945–10947
Yeh CH, Chen CH, Lin YC (2011) Use of a gradient-generating microfluidic device to rapidly determine a suitable glucose concentration for cell viability test. Microfluid Nanofluid 10:1011–1018
Acknowledgments
This work was supported by a grant from the National Research Foundation (NRF) of Korea (NRF-2009-C1AAA001-2009-0093479) funded by the Ministry of Education, Science, and Technology by a grant from the Next-Generation BioGreen 21 program (SSAC, No. PJ00954905), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, M., Jia, M., Kim, Y. et al. Rapid and accurate generation of various concentration gradients using polydimethylsiloxane-sealed hydrogel device. Microfluid Nanofluid 16, 645–654 (2014). https://doi.org/10.1007/s10404-013-1265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-013-1265-y