Abstract
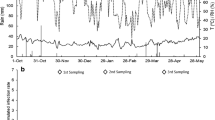
Flavescence dorée (FD) is one of the most economically important grapevine diseases in Southern Europe, and it is associated with phytoplasmas, phloem-limited wall-less bacteria. Recovery from disease naturally occurs in infected grapevines during the following seasons after infection. The capability of the leafhopper vector Scaphoideus titanus to acquire FD phytoplasma (FDP) from recovered and infected grapevines of Barbera and Nebbiolo varieties was investigated in North-western Italy vineyards monitored from 2007 to 2011. Pathogen concentration was quantified by real-time PCR in FDP-infected grapevines and broad beans, also used as source plants under controlled conditions, to correlate acquisition capabilities and phytoplasma titre in source plants. S. titanus acquired FDP from infected, but not from recovered, grapevines. FDP titre was higher in Barbera than in Nebbiolo and higher in summer than in spring, and acquisition efficiency and pathogen titre in source plants were positively correlated, both in field and laboratory conditions. Recovered plants do not represent a source of inoculum for the vector and therefore do not contribute to FDP spread. The inability of recovered plants to serve as FDP acquisition sources for the vector as well as the effect of the season and of the two grapevine varieties on the FDP acquisition efficiency are relevant results to re-design disease management practices, especially since insecticide treatments against the vector are not fully effective, and newly designed successful control strategies are required.


Similar content being viewed by others
References
Angelini E, Clair D, Borgo M, Bertaccini A, Boudon-Padieu E (2001) Flavescence dorée in France and Italy—occurrence of closely related phytoplasma isolates and their near relationships to Palatinate grapevine yellows and an alder yellows phytoplasma. Vitis 40:79–86
Belli G, Bianco PA, Conti M (2010) Grapevine yellows in Italy: past, present and future. J Plant Pathol 92:303–326
Bellomo C, Carraro L, Ermacora P, Pavan F, Osler R, Frausin C, Governatori G (2007) Recovery phenomena in grapevines affected by grapevine yellows in Friuli Venezia Giulia. Bull Insectol 60:235–236
Bosio G, Rossi A (2001) Ciclo biologico in Piemonte di Scaphoideus titanus. L’Informatore Agrario 21:75–78
Bressan A, Spiazzi S, Girolami V, Boudon-Padieu E (2005) Acquisition efficiency of Flavescence dorée phytoplasma by Scaphoideus titanus Ball from infected tolerant or susceptible grapevine cultivars or experimental host plants. Vitis 44:143–146
Camussi A, Möller F, Ottaviano E, Sari Gorla M (1995) Metodi statistici per la sperimentazione biologica, II edn. Zanichelli, Torino, p 496
Caudwell A, Kuszala C, Larrue J, Bachelier JC (1972) Transmission de la Flavescence dorée de la fève à la fève par des cicadelles des genres Euscelis et Euscelidius. Ann Phytopathol No. hors série 181–189
Caudwell A, Boudon-Padieu E, Kuszala C, Larrue J (1987) Biologie et étiologie de la Flavescence dorée. Recherches sur son diagnostic et sur les méthodes de lutte.In: Proceedings of the conference on grapevine Flavescence dorée, Vicenza Verona, Italy, May 28–29, pp 175–208
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129
Feil H, Feil WS, Purcell AH (2003) Effects of date of inoculation on the within-plant movement of Xylella fastidiosa and persistence of Pierce’s disease within field grapevines. Phytopathology 93:244–251
Foissac X, Wilson MR (2010) Current and possible future distributions of phytoplasma diseases and their vectors. In: Weintraub PG, Jones P (eds) Phytoplasmas: genomes. Plant hosts and vectors, CABI. Wallingford, pp 309–324
Galetto L, Bosco D, Marzachì C (2005) Universal and group-specific real-time PCR diagnosis of flavescence dorée (16Sr-V), bois noir (16Sr-XII) and apple proliferation (16Sr-X) phytoplasmas from field-collected plant hosts and insect vectors. Ann Appl Biol 147:191–201
Gambino G, Boccacci P, Margaria P, Palmano S, Gribaudo I (2013) Hydrogen peroxide accumulation and transcriptional changes in grapevines recovered from Flavescence dorée disease. Phytopathology 103:776–784. doi:10.1094/phyto-11-12-0309-r
Garau R, Tolu G, Prota VA, Sechi A (2004) Differential reactivity of grapevine cultivars to “Bois noir” infections in Sardinia. J Plant Pathol 86:320
Garau R, Sechi A, Prota VA, Moro G (2007) Productive parameters in Chardonnay and Vermentino grapevines infected with “bois noir” and recovered in Sardinia. Bull Insectol 60:233–234
Hogenhout SA, Oshima K, Ammar ED, Kakizawa S, Kingdom HN, Namba S (2008) Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9:403–423
Lee IM, Gundersen DE, Hammond RW, Davis RE (1994) Use of mycoplasmalike organism (MLO) group-specific oligonucleotide primers for nested-PCR assays to detect mixed-MLO infections in a single host-plant. Phytopathology 84:559–566
Lherminier J, Courtois M, Caudwell A (1994) Determination of the distribution and multiplication sites of Flavescence dorée mycoplasma-like organisms in the host plant Vicia faba by ELISA and immunocytochemistry. Physiol Mol Plant Pathol 45:125–138
Margaria P, Palmano S (2011) Response of the Vitis vinifera L. cv. ‘Nebbiolo’ proteome to Flavescence dorée phytoplasma infection. Proteomics 11:212–224. doi:10.1002/pmic.201000409
Margaria P, Abbà S, Palmano S (2013) Novel aspects of grapevine response to phytoplasma infection investigated by a proteomic and phospho-proteomic approach with data integration into functional networks. BMC Genomics 14:38. doi:10.1186/1471-2164-14-38
Martini M, Botti S, Marcone C, Marzachì C, Casati P, Bianco PA, Benedetti R, Bertaccini A (2002) Genetic variability among Flavescence dorée phytoplasmas from different origins in Italy and France. Mol Cell Probes 16:197–208
Marzachì C, Bosco D (2005) Relative quantification of chrysanthemum yellows (16SrI) phytoplasma in its plant and insect host using real-time polymerase chain reaction. Mol Biotechnol 30:117–127
Mori N, Pavan F, Reggiani N, Bacchiavini M, Mazzon L, Paltrinieri S, Bertaccini A (2012) Correlation of bois noir disease with nettle and vector abundance in northern Italy vineyards. J Pest Sci 85:23–28
Morone C, Boveri M, Giosué S, Gotta P, Rossi V, Scapin I, Marzachì C (2007) Epidemiology of Flavescence dorée in vineyards in northwestern Italy. Phytopathology 97:1422–1427
Musetti R, Di Toppi LS, Ermacora P, Favali MA (2004) Recovery in apple trees infected with the apple proliferation phytoplasma: an ultrastructural and biochemical study. Phytopathology 94:203–208
Musetti R, di Toppi LS, Martini M, Ferrini F, Loschi A, Favali MA, Osler R (2005) Hydrogen peroxide localization and antioxidant status in the recovery of apricot plants from European stone fruit yellows. Eur J Plant Pathol 112:53–61
Musetti R, Marabottini R, Badiani M, Martini M, di Toppi LS, Borselli S, Borgo M, Osler R (2007) On the role of H2O2 in the recovery of grapevine (Vitis vinifera cv. Prosecco) from Flavescence dorée disease. Funct Plant Biol 34:750–758
Osler R, Carraro L, Ermacora P, Ferrini F, Loi N, Loschi A, Martini M, Mutton PB, Refatti R (2003) Roguing: a controversial practice to eradicate grape yellows caused by phytoplasmas. In: Proceedings of the 14th meeting of the international council for the study of virus and virus–like diseases of the grapevine (ICVG), Locorotondo (BA), Italy, Sep 12–17, pp 68
Osler R, Borselli S, Ermacora P, Loschi A, Martini M, Musetti R, Loi N (2014) Acquired tolerance in apricot plants that stably recovered from European stone fruit yellows. Plant Dis 98:492–496
Pavan F, Mori N, Bigot G, Zandigiacomo P (2012) Border effect in spatial distribution of Flavescence dorée affected grapevines and outside source of Scaphoideus titanus vectors. Bull Insectol 65:281–290
Roggia C, Caciagli P, Galetto L, Pacifico D, Veratti F, Bosco D, Marzachì C (2013) Flavescence dorée phytoplasma titre in field-infected Barbera and Nebbiolo grapevines. Plant Pathol 63:31–41. doi:10.1111/ppa.12068
Romanazzi G, Murolo S (2008) Partial uprooting and pulling to induce recovery in Bois noir-infected grapevines. J Phytopathol 156:747–750. doi:10.1111/j.1439-0434.2008.01424.x
Romanazzi G, D’Ascenzo D, Murolo S (2009) Field treatment with resistance inducers for the control of grapevine Bois noir. J Plant Pathol 91:677–682
Romanazzi G, Murolo S, Feliziani E (2013) Effects of an innovative strategy to contain grapevine bois noir: field treatment with resistance inducers. Phytopathology 103:785–791. doi:10.1094/phyto-01-13-0031-r
Salar P, Charenton C, Foissac X, Malembic-Maher S (2013) Multiplication kinetics of Flavescence dorée phytoplasma in broad bean. Effect of phytoplasma strain and temperature. Eur J Plant Pathol 135:371–381. doi:10.1007/s10658-012-0093-3
Saracco P, Bosco D, Veratti F, Marzachì C (2006) Quantification over time of chrysanthemum yellows phytoplasma (16Sr-I) in leaves and roots of the host plant Chrysanthemum carinatum (Schousboe) following inoculation with its insect vector. Physiol Mol Plant Pathol 67:212–219
Schneider B, Seemüller E, Smart CD, Kirkpatrick BC (1995) Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In: Razin R, Tully JG (eds) Molecular and diagnostic procedures in mycoplasmology, vol I. Academic Press, San Diego, pp 369–380
Schvester D, Carle P, Montous G (1963) Transmission de la Flavescence dorée de la vigne par S. littoralis Ball. Ann Epiphyt 14:175–198
Seemüller E, Kunze L, Schaper U (1984) Colonization behaviour of MLO, and symptom expression of proliferation-diseased apple trees and decline-diseased pear trees over a period of several years. J Plant Dis Prot 91:525–532
Speich P, Méjean I, Noyer C, Thomas C, Gillet J, Cloquemin G, Clair D, Boudon-Padieu E (2006) Limited susceptibility of Syrah cv. to Flavescence dorée and Bois noir in South of France. In: Proceedings of the 15th meeting of the international council for the study of virus and virus-like diseases of the grapevine (ICVG), Stellenbosch, South Africa, April 3–7. pp 171–172
SPSS for Windows (2011) Version 20.0.0. IBM Corp
Swallow WH (1985) Group testing for estimating infection rates and probabilities of disease transmission. Phytopathology 75:882–889. doi:10.1094/Phyto-75-882
Untergrasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115
Vitali M, Chitarra W, Galetto L, Bosco D, Marzachì C, Gullino ML, Spanna F, Lovisolo C (2013) Flavescence dorée phytoplasma deregulates stomatal control of photosynthesis in Vitis vinifera. Ann Appl Biol 162:335–346. doi:10.1111/aab.12025
Acknowledgments
This research is funded by the Piemonte Region with the projects: ‘Adoption of a multidisciplinary approach to study the grapevine agroecosystem: analysis of biotic and abiotic factors able to influence yield and quality’, ‘Studi su fitoplasmi della vite e loro vettori: sensibilità varietale e efficienza di acquisizione di Flavescenza dorata, caratterizzazione, diffusione e vettori di Legno nero, tecniche di riduzione del danno’. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Galetto, L., Miliordos, D., Roggia, C. et al. Acquisition capability of the grapevine Flavescence dorée by the leafhopper vector Scaphoideus titanus Ball correlates with phytoplasma titre in the source plant. J Pest Sci 87, 671–679 (2014). https://doi.org/10.1007/s10340-014-0593-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0593-3