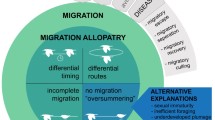
Abstract
All organisms are adapted to their native environment, posing problems in terms of fitness costs for individuals that leave this environment. Other species constitute an important coevolving component of this native environment, with more than half of all living organisms being parasites. All host species have evolved behavioral and physiological defenses against parasitism, and all higher organisms have evolved immunity that rely on exposure to novel substances derived from other organisms (antigens) for developing efficient defenses. The evolution of dispersal must have been affected by host–parasite interactions because dispersers and migrants inevitably encounter novel parasite strains to which they are not adapted. We provide an overview of bird migration and migratory connectivity viewed in the light of host–parasite interactions. Migratory birds generally show strong site fidelity to both breeding and wintering locations, as evidenced from studies of individuals, estimates of adult survival rates based on breeding or non-breeding captures, and studies of migratory connectivity. Connectivity is closely linked to development of the immune system and regression of immune defense organs. Empirical and theoretical evidence suggests that site fidelity is under stabilizing selection, and that offspring resemble their parents in terms of site fidelity. Populations may diverge in connectivity when the fitness benefits in terms of parasitism exceed the costs. Many species of birds have evolved migratory divides from glacial and post-glacial isolation that may constitute incipient speciation linked to divergence in parasite faunas and hence in local co-adaptation of hosts and parasites in the breeding and wintering quarters. Migration may play a role in speciation when interbreeding among hosts causes divergence in fitness costs and benefits of parasitism due to local adaptation in breeding and wintering areas. These ecological and evolutionary scenarios for migration and migratory connectivity provide a number of testable assumptions and predictions that can form the basis of future research.

Similar content being viewed by others
References
Alerstam T (1998) The development of bird migration theory. J Avian Biol 29:343–369
Alerstam T, Enckell PH (1979) Unpredictable habitats and evolution of bird migration. Oikos 33:228–232
Alerstam T, Lindström Å (1990) Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner E (ed) Bird migration: physiology and ecophysiology. Springer, Berlin, pp 331–351
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Ambrosini R, Møller AP, Saino N (2009) A quantitative measure of migratory connectivity. J Theor Biol 257:203–211
Balbontín J, Møller AP, Hermosell IG, Marzal A, Reviriego M, de Lope F (2009) Individual responses in spring arrival date to ecological conditions during winter and migration in a migratory bird. J Anim Ecol 78:981–989
Belliure J, Sorci G, Møller AP, Clobert J (2000) Dispersal distances predict subspecies richness in birds. J Evol Biol 13:480–487
Bensch S, Åkesson S (2003) Temporal and spatial variation of Haematozoan in Scandinavian willow warblers. J Parasitol 89:388–391
Berthold P (2001) Bird migration. Oxford University Press, Oxford
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. BirdLife International, Cambridge, UK
Chamberlain CP, Bensch S, Feng X, Åkesson S, Andersson T (2000) Stable isotopes examined across a migratory divide in Scandinavian willow warblers (Phylloscopus trochilus trochilus and Phylloscopus trochilus acredula) reflect their African winter quarters. Proc R Soc Lond B 267:43–48
Clobert J, Nichols JD, Danchin E, Dhondt A (eds) (2001) Dispersal. Oxford University Press, Oxford
Combes C (2001) Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press, Chicago
Cramp S, Perrins CM (eds) (1977–1994) The birds of the western Palearctic. Oxford University Press, Oxford
Durrant KL, Reed JL, Jones PJ, Dallimer N, Cheke RA, McWilliam AN, Fleischer RC (2007) Variation in haematozoan parasitism at local and landscape levels in the red-billed quelea Quelea quelea. J Avian Biol 38:662–671
Durrant KL, Marra PP, Fallon SM, Colbeck GJ, Gibbs HL, Hobson KA, Norris DR, Bernik B, Lloyd VL, Fleischer RC (2008) Parasite assemblages distinguish populations of a migratory passerine on its breeding grounds. J Zool 274:318–326
Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JRD (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci USA 107:2078–2081
Eppig C, Fincher CL, Thornhill R (2010) Parasite prevalence and the worldwide distribution of cognitive ability. Proc R Soc Lond B 277:3801–3808
Fallon S, Fleischer R, Graves G (2006) Malarial parasites as geographical markers in migratory birds? Biol Lett 2:213–216
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622
Glutz von Blotzheim UN, Bauer KM (eds) (1966–1997) Handbuch der Vögel Mitteleuropas. Aula, Wiesbaden
Greenwood PJ, Harvey PH (1982) Natal and breeding dispersal in birds. Annu Rev Ecol Syst 13:1–21
Guégan J-F, Thomas F, Hochberg M, de Meeus T, Renaud F (2001) Disease diversity and human fertility. Evolution 55:1308–1314
Guernier V, Hochberg ME, Guégan J-F (2004) Ecology drives the worldwide distribution of human diseases. PLoS Biol 2:740–746
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Herrera C (1978) Breeding distribution pattern of European migrant birds: MacArthur theme reexamined. Auk 95:496–509
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hjernquist MB, Veen T, Font L, Klaassen M (2009) High individual repeatability and population differentiation in stable isotope ratios in winter-grown collared flycatcher Ficedula albicollis feathers. J Avian Biol 40:102–107
Hobson KA (2008) Using endogenous and exogenous markers in bird conservation. Bird Cons Int 18:S174–S199
Hobson KA, Wassenaar LI (eds) (2008) Tracking animal migration with stable isotopes. Academic, London
Kaltz O, Shykoff JA (1998) Local adaptation in host-parasite systems. Heredity 81:361–370
Kelsey MG (1989) A comparison of the song and territorial behaviour of a long-distance migrant, the marsh warbler Acrocephalus palustris, in summer and winter. Ibis 131:403–414
Lack D (1944) Ecological aspects of species-formation in passerine birds. Ibis 86:260–286
Lindström Å (1990) The role of predation risk in stopover habitat selection in migrating bramblings, Fringilla montifringilla. Behav Ecol 1:102–106
Lindström Å, Alerstam T (1992) Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am Nat 140:477–491
MacArthur RH (1959) On the breeding distribution pattern of North American migrant birds. Auk 76:318–325
MacArthur RH (1972) Geographical ecology. Harper and Row, New York
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886
Mayr E (1942) Systematics and the origin of species. Columbia University Press, New York
McKinnon L, Smith PA, Nol E, Martin JL, Doyle FI, Abraham KF, Gilchrist HG, Morrison RIG, Bêty J (2010) Lower predation risk for migratory birds at high latitudes. Science 327:326–327
Mendes L, Piersma T, Lecoq M, Spaans B, Ricklefs RE (2005) Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos 109:396–404
Møller AP (2008) Interactions between interactions: predator-prey, parasite-host and mutualistic interactions. N Y Acad Sci 1133:180–186
Møller AP (2010) Brain size, head size and behavior of a passerine bird. J Evol Biol 23:625–635
Møller AP, Erritzøe J (2001) Dispersal, vaccination and regression of immune defence organs. Ecol Lett 4:484–490
Møller AP, de Lope F, Saino N (2004a) Parasitism, immunity and arrival date in a migratory bird. Ecology 85:206–219
Møller AP, Martin-Vivaldi M, Soler JJ (2004b) Parasitism, host immune response and dispersal. J Evol Biol 17:603–612
Møller AP, Erritzøe J, Garamszegi LZ (2005) Coevolution between brain size and immunity in birds: Implications for brain size evolution. J Evol Biol 18:223–237
Møller AP, Martin-Vivaldi M, Merino S, Soler JJ (2006) Density-dependent and geographical variation in bird immune response. Oikos 115:463–474
Møller AP, Arriero E, Lobato E, Merino S (2009) A meta-analysis of parasite virulence in nestling birds. Biol Rev 84:567–588
Morjan CL, Rieseberg LH (2004) How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol Ecol 13:1341–1356
Newton I (2008) The migration ecology of birds. Academic, London
Norris DR, Marra PP, Kyser TK, Montgomerie R, Ratcliffe LM (2004) Reproductive effort, molting latitude and feather color in a migratory songbird. Science 306:2249–2250
Pagenkopp KM, Klicka J, Durrant KL, Garvin JC, Fleischer RC (2008) Geographic variation in malarial parasite lineages in the common yellowthroat (Geothlypis trichas). Cons Genet 9:1577–1588
Paradis E, Baillie SR, Sutherland WJ, Gregory RD (1998) Patterns of natal and breeding dispersal in birds. J Anim Ecol 67:518–536
Peach WJ, Hanmer DB, Oatley TB (2001) Do southern African songbirds live longer than their European counterparts? Oikos 93:235–249
Phillimore AB, Freckleton RP, Orme CDL, Owens IPF (2006) Ecology predicts large-scale patterns of phylogenetic diversification in birds. Am Nat 168:220–229
Piersma T (1997) Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80:623–631
Procházka P, Stokke BG, Jensen H, Fainová D, Bellinvia E, Fossøy F, Vikan JR, Bryja J, Soler M (2010) Low genetic differentiation in a long-distance migratory bird across Europe. Biol J Linn Soc (in press)
Rensch B (1933) Zoologische Systematik und Artbildungsprobleme. Verh Dtsch Zool Ges 1933:19–83
Ricklefs RE, Outlaw DC (2010) A molecular clock for malaria parasites. Science 329:226–229
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65:514–527
Rolshausen G, Segelbacher G, Hobson KA, Schaefer HM (2009) Contemporary evolution of reproductive isolation and phenotypic divergence in sympatry along a migratory divide. Curr Biol 19:2097–2101
Saino N, Szép T, Ambrosini R, Romano M, Møller AP (2004) Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proc R Soc Lond B 271:681–686
Salomonsen F (1955) The evolutionary significance of bird-migration. Biol Medd K Dan Vidensk Selsk 22(6):1–62
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Sillett TS, Holmes RT, Sherry TW (2002) Impacts of a global climate cycle on population dynamics of a migratory songbird. Science 288:2040–2042
Snoeijs T, Van de Casteele T, Adriaensen F, Matthysen E, Eens M (2004) A strong association between immune responsiveness and natal dispersal in a songbird. Biol Lett 271:S199–S201
Sutherland WJ (1998) Evidence for flexibility and constraint in migration systems. J Avian Biol 29:441–446
Svensson LM, Ruegg KC, Sekercioglu CH, Sehgal RN (2007) Widespread and structured distributions of blood parasite haplotypes across a migratory divide of the Swainson’s thrush (Catharus ustulatus). J Parasitol 93:1488–1495
Szép T, Møller AP, Vallner J, Kovács B, Norman D (2003) Use of trace elements in feathers of sand martin Riparia riparia for identifying moulting areas. J Ornithol 34:307–320
Szép T, Hobson KA, Vallner J, Kovács B, Szabó DZ, Møller AP (2009) Comparison of trace element and stable isotope approaches to the study of migratory connectivity: An example using two hirundine species breeding in Europe and wintering in Africa. J Ornithol 150:621–636
von Rönn J (2010) Migration and blood parasites in barn swallows. PhD thesis, Max-Planck-Institute for Evolutionary Biology, Plön
Voříšek P (2008) Trends of common birds in Europe, 2008 update, computation procedure and data quality control in details. EBCC, Pargue, Czech Republic. URL: http://www.ebcc.info/index.php?ID=362. Accessed 14 Oct 2009
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83
Wilson CG, Sherman PW (2010) Anciently asexual bdelloid rotifers escape lethal fungal parasites by drying up and blowing away. Science 327:574–576
Zink G, Bairlein F (1987–1995) Der Zug europäischer Singvögel, vols 1–3. Aula, Wiesbaden
Acknowledgments
J. von Rönn kindly allowed us to cite his research. T.Sz. is grateful for the OTKA 69068 grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Møller, A.P., Szép, T. The role of parasites in ecology and evolution of migration and migratory connectivity. J Ornithol 152 (Suppl 1), 141–150 (2011). https://doi.org/10.1007/s10336-010-0621-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-010-0621-x