Abstract
Objective
The purpose of this study was to assess changes in the tumor microvasculature induced by combination antiangiogenic therapy in MCF-7 breast tumor mouse models, using a noninvasive DCE-MRI method that minimizes the effect of water exchange.
Materials and methods
3D quantitative DCE-MRI images were acquired with a heavily T 1-weighted saturation recovery gradient echo sequence with a recovery delay of 20 ms. Tumor vascular volume (VV) and vascular permeability-surface area product (PS) were obtained through a linear regression of the albumin-Gd-DTPA-enhanced dynamic image intensity on MCF-7 breast tumor mouse models treated with combination bevacizumab/paclitaxel therapy.
Results
Measured tumor VV values were significantly higher than the values that have been reported previously using quantitative T 1 mapping, and are in good agreement with micro-CT (computed tomography) results reported earlier from other tumor models. A trend of decreasing tumor PS was detected in the group of MCF-7 tumor bearing mice treated with the bevacizumab/paclitaxel combination regimen.
Conclusion
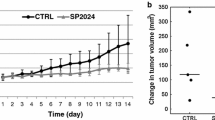
VV and PS maps obtained by a heavily T 1-weighted acquisition protocol revealed the large peripheral blood vessels as well as the permeable areas within the tumor. A 12-day/three-dose combination treatment of bevacizumab and paclitaxel resulted in delayed tumor growth and a trend of decreasing tumor vascular permeability surface area product.







Similar content being viewed by others
References
O’Connor JP, Jackson A, Parker GJ, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96:189–195
Preda A, Novikov V, Moglich M, Turetschek K, Shames DM, Brasch RC, Cavagna FM, Roberts TP (2004) MRI monitoring of Avastin antiangiogenesis therapy using B22956/1, a new blood pool contrast agent, in an experimental model of human cancer. J Magn Reson Imaging 20:865–873
Hylton N (2006) Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol 24:3293–3298
Heilmann M, Kiessling F, Enderlin M, Schad LR (2006) Determination of pharmacokinetic parameters in DCE MRI: consequence of nonlinearity between contrast agent concentration and signal intensity. Invest Radiol 41:536–543
Schabel MC, Parker DL (2008) Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol 53:2345–2373
Buckley DL, Parker GJ (2005) Measuring contrast agent concentration in T1-weighted dynamic contrast-enhanced MRI. In: Jackson A, Buckley DL, Parker GJ (eds) Dynamic contrast-enhanced magnetic resonance imaging in oncology, diagnostic imaging. Springer, Berlin, pp 69–79
Donahue KM, Weisskoff RM, Chesler DA, Kwong KK, Bogdanov AA Jr, Mandeville JB, Rosen BR (1996) Improving MR quantification of regional blood volume with intravascular T1 contrast agents: accuracy, precision, and water exchange. Magn Reson Med 36:858–867
Li X, Huang W, Rooney WD (2012) Signal-to-noise ratio, contrast-to-noise ratio and pharmacokinetic modeling considerations in dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 30:1313–1322
Burstein HJ (2011) Bevacizumab for advanced breast cancer: all tied up with a RIBBON? J Clin Oncol 29:1232–1235
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS (2011) RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 29:4286–4293
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Bahri S, Chen JH, Mehta RS, Carpenter PM, Nie K, Kwon SY, Yu HJ, Nalcioglu O, Su MY (2009) Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann Surg Oncol 16:1619–1628
Bhujwalla ZM, Artemov D, Natarajan K, Ackerstaff E, Solaiyappan M (2001) Vascular differences detected by MRI for metastatic versus nonmetastatic breast and prostate cancer xenografts. Neoplasia 3:143–153
Yanagisawa M, Yorozu K, Kurasawa M, Nakano K, Furugaki K, Yamashita Y, Mori K, Fujimoto-Ouchi K (2010) Bevacizumab improves the delivery and efficacy of paclitaxel. Anticancer Drugs 21:687–694
Oku N, Doi K, Namba Y, Okada S (1994) Therapeutic effect of adriamycin encapsulated in long-circulating liposomes on Meth-A-sarcoma-bearing mice. Int J Cancer 58:415–419
Ogan MD, Schmiedl U, Moseley ME, Grodd W, Paajanen H, Brasch RC (1987) Albumin labeled with Gd-DTPA. An intravascular contrast-enhancing agent for magnetic resonance blood pool imaging: preparation and characterization. Invest Radiol 22:665–671
Kato Y, Okollie B, Raman V, Vesuna F, Zhao M, Baker SD, Bhujwalla ZM, Artemov D (2007) Contributing factors of temozolomide resistance in MCF-7 tumor xenograft models. Cancer Biol Ther 6:891–897
Levitt MH (1982) Symmetrical composite pulses for NMR population inversion. I. Compensation of radiofrequency field inhomogeneity. J Magn Reson 48:234–264
Bhujwalla ZM, Artemov D, Natarajan K, Solaiyappan M, Kollars P, Kristjansen PE (2003) Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470. Clin Cancer Res 9:355–362
Donahue KM, Burstein D, Manning WJ, Gray ML (1994) Studies of Gd-DTPA relaxivity and proton exchange rates in tissue. Magn Reson Med 32:66–76
Vexler VS, Clement O, Schmitt-Willich H, Brasch RC (1994) Effect of varying the molecular weight of the MR contrast agent Gd-DTPA-polylysine on blood pharmacokinetics and enhancement patterns. J Magn Reson Imaging 4:381–388
Blasberg RG, Fenstermacher JD, Patlak CS (1983) Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab 3:8–32
Chen H, Li F, Zhao X, Yuan C, Rutt B, Kerwin WS (2011) Extended graphical model for analysis of dynamic contrast-enhanced MRI. Magn Reson Med 66:868–878
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Shames DM, Kuwatsuru R, Vexler V, Muhler A, Brasch RC (1993) Measurement of capillary permeability to macromolecules by dynamic magnetic resonance imaging: a quantitative noninvasive technique. Magn Reson Med 29:616–622
Roberts HC, Roberts TP, Brasch RC, Dillon WP (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Windberger U, Bartholovitsch A, Plasenzotti R, Korak KJ, Heinze G (2003) Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: reference values and comparison of data. Exp Physiol 88:431–440
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392
Bogin L, Degani H (2002) Hormonal regulation of VEGF in orthotopic MCF7 human breast cancer. Cancer Res 62:1948–1951
Wall A, Persigehl T, Hauff P, Licha K, Schirner M, Muller S, von Wallbrunn A, Matuszewski L, Heindel W, Bremer C (2008) Differentiation of angiogenic burden in human cancer xenografts using a perfusion-type optical contrast agent (SIDAG). Breast Cancer Res BCR 10:R23
Pathak AP, Artemov D, Ward BD, Jackson DG, Neeman M, Bhujwalla ZM (2005) Characterizing extravascular fluid transport of macromolecules in the tumor interstitium by magnetic resonance imaging. Cancer Res 65:1425–1432
Cheng HL, Wright GA (2006) Rapid high-resolution T(1) mapping by variable flip angles: accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med 55:566–574
Fournier LS, Novikov V, Lucidi V, Fu Y, Miller T, Floyd E, Shames DM, Brasch RC (2007) MR monitoring of cyclooxygenase-2 inhibition of angiogenesis in a human breast cancer model in rats. Radiology 243:105–111
Sennino B, Raatschen HJ, Wendland MF, Fu Y, You WK, Shames DM, McDonald DM, Brasch RC (2009) Correlative dynamic contrast MRI and microscopic assessments of tumor vascularity in RIP-Tag2 transgenic mice. Magn Reson Med 62:616–625
Savai R, Langheinrich AC, Schermuly RT, Pullamsetti SS, Dumitrascu R, Traupe H, Rau WS, Seeger W, Grimminger F, Banat GA (2009) Evaluation of angiogenesis using micro-computed tomography in a xenograft mouse model of lung cancer. Neoplasia 11:48–56
Kim E, Cebulla J, Ward BD, Rhie K, Zhang J, Pathak AP (2012) Assessing breast cancer angiogenesis in vivo: which susceptibility contrast MRI biomarkers are relevant. Magn Reson Med
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N (2008) Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci 49:522–527
Nagy JA, Dvorak HF (2012) Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis 29:657–662
Goldfarb SB, Traina TA, Dickler MN (2010) Bevacizumab for advanced breast cancer. Womens Health (Lond Engl) 6:17–25
Smith IE, Pierga JY, Biganzoli L, Cortes-Funes H, Thomssen C, Pivot X, Fabi A, Xu B, Stroyakovskiy D, Franke FA, Kaufman B, Mainwaring P, Pienkowski T, De Valk B, Kwong A, Gonzalez-Trujillo JL, Koza I, Petrakova K, Pereira D, Pritchard KI (2011) First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2,251 patients. Ann Oncol 22:595–602
von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Gerber B, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Fehm T, Muller BM, Denkert C, Loibl S, Nekljudova V, Untch M (2012) Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 366:299–309
Acknowledgments
This study was supported by NIH/NCI P50CA103175, R01CA154738, and a GlaxoSmithKline research award. We would like to thank Dr. Arvind P. Pathak, Dr. Susanta K. Sarkar, and Dr. Zaver M. Bhujwalla for helpful discussions. We are also grateful to Mr. Gary Cromwell for mouse tumor inoculation, Dr. Noriko Mori for the preparation of the albumin-Gd-DTPA used in this study, and Ms. Mary McAllister for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, W., Kato, Y. & Artemov, D. Water exchange-minimizing DCE-MRI protocol to detect changes in tumor vascular parameters: effect of bevacizumab/paclitaxel combination therapy. Magn Reson Mater Phy 27, 161–170 (2014). https://doi.org/10.1007/s10334-013-0389-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-013-0389-0