Abstract
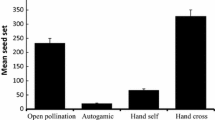
Reproductive strategies can have significant consequences for the viability of plant populations. Still, the effects of lower fruit set due to pollen limitation on plant demography and population persistence have rarely been explored. The objectives of this study were to assess the ecological factors determining female reproductive success and to study the impact of pollen limitation on population growth of Dracocephalum austriacum L. (Lamiaceae), a critically endangered species with a discontinuous distribution across Europe. Despite the significant background information gathered on the population dynamics and genetic diversity of D. austriacum, little is known about its reproductive strategy and the effect it has on population growth. Thus, the reproductive system, pollinator assemblage and pollen limitation were studied in natural populations and the impact of pollen-limited seed production on population growth was assessed using existing transition matrix models. The results revealed that D. austriacum is protandrous self-compatible species that produces very few seeds in the absence of pollinators. The flowers are visited by several insects, including legitimate pollinators (e.g., Bombus hortorum, Osmia spp.) and nectar robbers (other Bombus spp., O. aurulenta). Fruit and seed production was significantly pollen-limited in all populations studied. However, despite the positive effect of pollen supplementation on seed production, the resulting increase in seed number did not significantly increase population growth rates in any of the studied populations. Hence, we conclude that populations are demographically stable and current natural seed production is sufficient for the species’ persistence.




Similar content being viewed by others
References
Andrello M, Nicolè F, Till-Bottraud I, Gaggiotti OE (2012) Effect of stage-specific vital rates on population growth rates and effective population sizes in an endangered iteroparous plant. Conserv Biol 26:208–217
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, Dudash MR, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Bierzychudek P (1981) Pollinator limitation of plant reproductive effort. Am Nat 117:838–840
Bierzychudek P (1982) The demography of jack-in-the-pulpit, a forest perennial that changes sex. Ecol Monogr 52:335–351
Bond WJ (1994) Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philos Trans R Soc B Biol Sci 344:83–90
Bonin A, Nicolè F, Pompanon F, Miaud C, Taberlet P (2007) Population adaptive index: a new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Conserv Biol 21:697–708
Burd M (1994) Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Calvo RN (1993) Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74:1033–1042
Calvo RN, Horvitz CC (1990) Pollinator limitation, cost of reproduction, and fitness in plants: a transition matrix demographic approach. Am Nat 136:499–516
Castro S, Silveira P, Navarro L (2008) Consequences of nectar robbing for the fitness of a threatened plant species. Plant Ecol 199:201–208
Caswell H (2001) Matrix population models: construction, analysis, and interpretation. Sinauer Associates, Sunderland
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst 18:237–268
Classen-Bockhoff R (2007) Floral construction and pollination biology in the Lamiaceae. Ann Bot Lond 100:359–360
Council of European Communities (1992) Council Directive 92/43/EEC of 21 May on the conservation of natural habitats and of wild fauna and flora. Off J Eur Commun 35:7–50
Dafni A, Kevan P, Husband BC (2005) Practical pollination biology. Enviroquest Ltr, Cambridge
Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Environ Res 28:137–167
Dostálek T, Münzbergová Z (2013) Comparative population biology of critically endangered Dracocephalum austriacum L. in two distant regions. Folia Geobot 48:75–93
Dostálek T, Münzbergová Z, Plačková I (2010) Genetic diversity and its effect on fitness in an endangered plant species, Dracocephalum austriacum L. Conserv Genet 11:773–783
Dudash M (1990) Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution 44:1129–1139
Dudash M, Fenster C (2000) Inbreeding and outbreeding depression in fragmented populations. In: Young A, Clarke G (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 35–53
Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou P-O, Goodwillie C, Johnston MO, Kelly JK, Moeller DA, Porcher E, Ree RH, Vallejo-Marin M, Winn AA (2010) Plant mating systems in a changing world. Trends Ecol Evol 25:35–43
Ehrlén J, Eriksson O (1995) Pollen limitation and population growth in a herbaceous perennial legume. Ecology 76:652–656
Feldman TS, Morris WF (2011) Higher survival at low density counteracts lower fecundity to obviate Allee effects in a perennial plant. J Ecol 99:1162–1170
Fenster C, Galloway L (2001) Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata (Fabaceae). Conserv Biol 14:1406–1412
Fernández JD, Bosch J, Nieto-Ariza B, Gómez JM (2012) Pollen limitation in a narrow endemic plant: geographical variation and driving factors. Oecologia 170:421–431
Fishbein M, Venable DL (1996) Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77:1061–1073
Franco M, Silvertown J (2004) A comparative demography of plants based upon elasticities of vital rates. Ecology 85:531–538
Galloway LF (2001) The effect of maternal and paternal environments on seed characters in the herbaceous plant Campanula americana (Campanulaceae). Am J Bot 88:832–840
García MB, Ehrlén J (2002) Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. Am J Bot 89:1295–1302
Goodwillie C, Kalisz S, Eckert CG (2005) The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst 36:47–79
Griffin SR, Barrett SCH (2002) Factors affecting low seed: ovule ratios in a spring woodland herb, Trillium grandiflorum (Melanthiaceae). Int J Plant Sci 163:581–590
Haig D, Westoby M (1988) On limits to seed production. Am Nat 131:757–759
Herrera CM (1989) Pollinator abundance, morphology, and flower visitation rate: analysis of the quantity component in a plant–pollinator system. Oecologia 80:241–248
Herrera CM, Cerdá X, García MB, Guitián J, Medrano M, Rey PJ, Sánchez-Lafuente AM (2002) Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. J Evol Biol 15:108–121
Holub J, Procházka F (2000) Red list of vascular plants of the Czech Republic. Preslia 72:187–230
Horvitz CC, Ehrlén J, Matlaga D (2010) Context-dependent pollinator limitation in stochastic environments: can increased seed set overpower the cost of reproduction in an understorey herb? J Ecol 98:268–278
Hrouda L (2002) Dracocephalum austriacum L. In: Kubát K, Hrouda L, Chrtek JJ, Kaplan Z, Kirschner J, Štěpánek J (eds) Klíč ke květeně České republiky (Key to the flora of the Czech Republic). Academia, Prague, p 592
Inouye DW (1980) The terminology of floral larceny. Ecology 61:1251–1253
Irwin RE, Bronstein JL, Manson JS, Richardson L (2010) Nectar Robbing: ecological and evolutionary perspectives. Annu Rev Ecol Evol Syst 41:271–292
IUCN (2012) IUCN red list of threatened species, version 2012.2. http://www.iucnredlist.org. Accessed September 2012
Knight TM (2004) The effect of herbivory and pollen limitation on a declining population of Trillium grandiflorum. Ecol Appl 14:915–928
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman TL (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Lande R (1998) Anthropogenic, ecological and genetic factors in extinction and conservation. Res Popul Ecol 40:259–269
Larson BMH, Barrett SCH (2000) A comparative analysis of pollen limitation in flowering plants. Biol J Linnean Soc 69:503–520
Law W, Salick J, Knight TM (2010) The effects of pollen limitation on population dynamics of snow lotus (Saussurea medusa and S. laniceps, Asteraceae): threatened Tibetan medicinal plants of the eastern Himalayas. Plant Ecol 210:343–357
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952
Lennartsson T (2002) Extinction thresholds and disrupted plant–pollinator interactions in fragmented plant populations. Ecology 83:3060–3072
Meusel H, Jäger E, Rauschert S, Weinert E (1978) Vergleichende Chorologie der zentraleuropäischen Flora—Karten. Gustav Fischer Verlag, Jena (in German)
Milberg P, Bertilsson A (1997) What determines seed set in Dracocephalum ryuschiana L. an endangered grassland plant. Flora 192:361–367
Moeller DA (2005) Pollinator community structure and sources of spatial variation in plant–pollinator interactions in Clarkia xantiana ssp. xantiana. Oecologia 142:28–37
Morgan MT, Schoen DJ, Bataillon TM (1997) The evolution of self-fertilization in perennials. Am Nat 150:618–638
Münzbergová Z (2005) Determinants of species rarity: population growth rates of species sharing the same habitat. Am J Bot 92:1987–1994
Münzbergová Z (2006) Effect of population size on the prospect of species survival. Folia Geobot 41:137–150
Münzbergová Z (2007) Population dynamics of diploid and hexaploid populations of a perennial herb. Ann Bot Lond 100:1259–1270
Navarro L (1997) Is the dichogamy of Salvia verbenaca (Lamiaceae) an effective barrier to self-fertilization? Plant Syst Evol 207:111–117
Nicolè F, Dahlgren JP, Vivat A, Till-Bottraud I, Ehrlén J (2011) Interdependent effects of habitat quality and climate on population growth of an endangered plant. J Ecol 99:1211–1218
Oostermeijer JGB (2000) Population viability analysis of the rare Gentiana pneumonanthe: the importance of genetics, demography and reproductive biology. In: Young A, Clarke G (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 313–334
Oostermeijer JGB (2003) Threats to rare plant persistence. In: Brigham C, Schwartz M (eds) Population viability in plants: conservation, management, and modeling of rare plants. Springer-Verlag, Heidelberg, pp 17–58
Parker IM (1997) Pollinator limitation of Cytisus scoparius (Scotch broom), an invasive exotic shrub. Ecology 78:1457–1470
Price MV, Campbell DR, Waser NM, Brody AK (2008) Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology 89:1596–1604
Raabová J, Münzbergová Z, Fischer M (2009) Consequences of near and far between-population crosses for offspring fitness in a rare herb. Plant Biol 11:829–836
Ramula S (2008) Population dynamics of a monocarpic thistle: simulated effects of reproductive timing and grazing of flowering plants. Acta Oecol 33:231–239
Sales F, Hedge IC, Christie F (2010) Salvia plebeia R. Br.: taxonomy, phytogeography, autogamy and myxospermy. Pak J Bot 42:99–110
Tomimatsu H, Ohara M (2006) Evaluating the consequences of habitat fragmentation: a case study in the common forest herb Trillium camschatcense. Popul Ecol 48:189–198
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Wesselingh RA (2007) Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174:26–37
Willi Y, van Kleunen M, Dietrich S, Fischer M (2007) Genetic rescue persists beyond first-generation outbreeding in small populations of a rare plant. Proc R Soc B Biol Sci 274:2357–2364
Zhang Y-W, Zhao J-M, Wang Y (2011) The dynamics of pollen removal and deposition, and its effects on sexual phases in a protandrous plant: Glechoma longituba (Lamiaceae). Nord J Bot 29:105–111
Acknowledgments
This study was supported by GAČR P505/10/0593, postdoc GAČR project 13-10850P, by a long-term research development project no. RVO 67985939, institutional project MŠMT and by FCT and European Social Fund with the fellowship FCT/BPD/41200/2007 and IF/01267/2013 Starting grant to SC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, S., Dostálek, T., van der Meer, S. et al. Does pollen limitation affect population growth of the endangered Dracocephalum austriacum L.?. Popul Ecol 57, 105–116 (2015). https://doi.org/10.1007/s10144-014-0458-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-014-0458-x