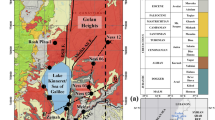
Abstract
The geochemical and isotopic composition of surface waters and groundwater in the Velenje Basin, Slovenia, was investigated seasonally to determine the relationship between major aquifers and surface waters, water–rock reactions, relative ages of groundwater, and biogeochemical processes. Groundwater in the Triassic aquifer is dominated by HCO3 –, Ca2+, Mg2+ and δ13CDIC indicating degradation of soil organic matter and dissolution of carbonate minerals, similar to surface waters. In addition, groundwater in the Triassic aquifer has δ18O and δD values that plot near surface waters on the local and global meteoric water lines, and detectable tritium, likely reflecting recent (<50 years) recharge. In contrast, groundwater in the Pliocene aquifers is enriched in Mg2+, Na+, Ca2+, K+, and Si, and has high alkalinity and δ13CDIC values, with low SO4 2– and NO3 – concentrations. These waters have likely been influenced by sulfate reduction and microbial methanogenesis associated with coal seams and dissolution of feldspars and Mg-rich clay minerals. Pliocene aquifer waters are also depleted in 18O and 2H, and have 3H concentrations near the detection limit, suggesting these waters are older, had a different recharge source, and have not mixed extensively with groundwater in the Triassic aquifer.
Résumé
La composition géochimique et isotopique de l’eau de surface et de l’eau souterraine dans le bassin de Velenje en Slovénie a été étudiée en fonction des saisons pour déterminer la relation entre les aquifèreres principaux et les eaux de surface, les interactions eau–roche, l’âge relatif de l’eau et les processus biogéochimiques. L’eau souterraine dans l’aquifère triasique est dominée par HCO3 –, Ca2+, Mg2+ and δ13CDIC qui indiquent que la dégradation de la matière organique du sol et la dissolution des carbonates est similaire à celle de l’eau de surface. En outre, l’eau souterraine de l’aquifère triasique a des valeurs en δ18O et δD qui se positionnent près des eaux de surface sur les droites des eaux météoriques globale et locale, et du tritium détectable qui marque probablement une recharge récente (<50 ans). Au contraire, l’eau souterraine des aquifères pliocènes est enrichie en Mg2+, Na+, Ca2+, K+, et Si, et a une alcalinité et des valeurs de δ13CDIC élevées, ainsi que des concentrations faibles en SO4 2– et NO3 –. Ces eaux ont probablement été influencées par la réduction des sulfates et la méthanogénèse microbienne associée aux veines de charbon et à la dissolution des feldspaths et minéraux magnésiens argileux. Les eaux de l’aquifère pliocène sont également appauvries en 18O et 2H, et ont des concentrations en 3H proches de la limite de détection, ce qui suggère que ces eaux sont plus anciennes, ont une recharge d’origine différente et n’ont pas été largement mélangée avec les eaux de l’aquifère triasique.
Resumen
Las composición geoquímica e isotópica del agua superficial y el agua subterránea en la Velenje Basin, Eslovenia, fue investigada estacionalmente para determinar la relación entre los acuíferos principales y el agua superficial, las reacciones agua – roca, las edades relativas del agua subterránea y los procesos biogeoquímicos. El agua subterránea en el acuífero Triásico es dominada por HCO3 –, Ca2+, Mg2+ y δ13CDIC indicando una degradación de la materia orgánica del suelo y la disolución de minerales carbonáticos, similares a las aguas superficiales. Además, el agua subterránea en el acuífero Triásico tiene valores de δ18O y δD que se ubican cerca de las aguas superficiales y de la línea de agua meteórica local y global, y el tritio detectable, reflejando probablemente una recarga reciente (<50 años). En contraste, el agua subterránea en los acuíferos Pliocenos está enriquecida en Mg2+, Na+, Ca2+, K+, y Si,y tiene altos valores de alcalinidad y de δ13CDIC, con bajas concentraciones de SO4 2– y NO3 –. Estas aguas han sido probablemente influenciadas por una reducción de sulfatos y una metanogenesis microbiana asociadas con capas de carbón y disolución de feldespatos y minerales de arcillas ricos en Mg. Las aguas de acuíferos del Plioceno están también empobrecidas en 18O y 2H, y tienen concentraciones de 3H cerca del límite de detección, lo que sugiere que estas aguas son más viejas, tienen una fuente diferente de recarga, y no se han mezclado extensivamente con el agua subterránea del acuífero Triásico.
摘要
对斯洛文尼亚Velenje盆地-地表水和地下水地球化学和同位素组分进行了周期性调查,以确定主要含水层和地表水体之间的关系、水-岩作用、地下水的相对年龄及生物地球化学过程。三叠纪含水层中的 水主要受HCO3 –, Ca2+, Mg2+ 及 δ13CDIC控制,表明土壤有机物质的退化和碳酸盐矿物的溶解,类似于地表水体。另外,三叠纪含水层中地下水中的δ18O and δD值在局部和全球大气水线上地表水体附近,地下水中也有可检出的氚,可能反映出最近(小于50年)有补给。与此相反,上新世含水层中的地下水富含Mg2+, Na+, Ca2+, K+, 及 Si, 具有很高的碱度和δ13CDIC v 值,但SO4 2– and NO3 – c 含量低。这些水可能受到过与煤层和长石及富镁粘土矿物相关的硫酸盐还原和微生物甲烷生成的影响。上新世含水层水也缺少18O 和 2H, 3H含量接近检出限,表明这些水较老,有不同的补给源,没有与三叠纪含水层中的水广泛混合。
Resumo
A composição geoquímica e isotópica das águas superficiais e da água subterrânea na Bacia de Velenje, na Eslovénia, foi investigada sazonalmente, a fim de determinar a relação entre os aquíferos principais e as águas superficiais, as reações água–rocha, as idades relativas da água subterrânea e os processos biogeoquímicos. A água subterrânea no aquífero Triássico é dominada por HCO3 –, Ca2+, Mg2+ e δ13CDIC, indicando degradação da matéria orgânica do solo e dissolução dos minerais carbonatados, similarmente às águas superficiais. Em adição, a água subterrânea do aquífero Triássico tem valores de δ18O e δD que se localizam próximos dos das águas superficiais locais e da linha das águas meteóricas globais, e do trítio detetável, refletindo presumivelmente recarga recente (<50 anos). Em contraste, a água subterrânea dos aquíferos Pliocénicos é enriquecida em Mg2+, Na+, Ca2+, K+ e Si e apresenta valores de alcalinidade e de δ13CDIC elevados, com baixas concentrações de SO4 2– e NO3 –. Estas águas são provavelmente influenciadas pela redução de sulfatos e pela metanogénese microbiana associada a camadas de carvão e dissolução de feldspatos e de minerais argilosos ricos em Mg. As águas dos aquíferos Pliocénicos são também deficitárias em 18O e 2H, e têm concentrações de 3H próximas dos limites de deteção, sugerindo que estas águas são mais antigas, apresentam uma fonte de recarga diferente, e não se têm misturado extensivamente com a água subterrânea do aquífero Triássico.
Povzetek
Geokemično in izotopsko sestavo površinskih in podzemnih vod v Velenjskem bazenu, Slovenija smo sezonsko raziskali za določitev interakcij med glavnimi vodonosniki in površinskimi vodami, reakcij med vodo-kamnino, relativnimi starostmi vod in biogeokemijskimi procesi. V podzemni vodi Triasnega vodonosnika prevladuje HCO3 –, Ca2+, Mg2+ in δ13CDIC, kar kaže na razgradnjo preperinske organske snovi in raztapljanje karbonatnih mineralov, podobno kot površinske vode. Dodatno, podzemna voda v Triasnih vodonosnikih ima vrednosti δ18O in δD, ki se nahajajo blizu lokalne in globalne meteorne premice in meje zaznavnosti za tricij, kar nakazuje na recentno (<50 years) napajanje. Kontrastno, so podzemne vode v Pliocenskih vodonosnikih obogatene z Mg2+, Na+, Ca2+, K+ in Si in imajo visoko alkalnost in δ13CDIC vrednosti, z nizko koncentracijo SO4 2– in NO3 –. Te vode so podvržene sulfatni redukciji in mikrobni metanogenezi, ki je povezana s premogovimi plastmi in raztapljanjem glinencev in Mg-bogatih mineralov. Pliocenske podzemne vode so osiromašene z 18O in 2H in imajo 3H koncentracije blizu meje detekcije, kar kaže da so te vode starejše, imajo drug vir napajanja in se ne mešajo intenzivno s podzemno vodo Triasnega vodonosnika.






Similar content being viewed by others
References
Aravena R, Harrison SM, Barker JF, Abercrombie H, Rudolph D (2003) Origin of methane in the Elk Valley coalfield, southeastern British Columbia, Canada. Chem Geol 1–4:219–227
Atekwana EA, Krishnamurthy RV (1998) Seasonal variations of dissolved inorganic carbon and δ13C of surface waters: application of a modified gas evaluation technique. J Hydrol 205(3–4):260–278
Aucour AM, Sheppard SMF, Guyomar OJ, Wattelet J (1999) Use of 13C to trace origin and cycling of inorganic carbon in the Rhône River system. Chem Geol 159(1–4):87–105
Brezigar A, Ogorelec B, Rijavec L, Mioč P (1988) Geologic setting of the pre-Pliocene basement of the Velenje depression and its surroundings (in Slovene). Geologija (Ljubljana) 30(1987):31–65
Cartwright I (2010) Using groundwater geochemistry and environmental isotopes to assess the correction of 14C ages in a silicate-dominated aquifer system. J Hydrol 382:174–187
Cartwright I, Weaver T, Tweed S, Ahearne D, Cooper M, Czapnik C, Tranter J (2000) O, H, C isotope geochemistry of carbonated mineral springs in central Victoria, Australia: sources of gas and water–rock interactions during basaltic volcanism. J Geochem Explor 69–70(69/1):257–261
Cartwright I, Weaver T, Tweed S, Ahearne D, Cooper M, Czapnik K, Tranter J (2002) Stable isotope geochemistry of cold CO2-bearing mineral spring waters, Daylesford, Victoria, Australia: sources of gas and water and links with waning volcanism. Chem Geol 185:71–91
Cartwright I, Weaver TR, Cendόn DI, Fifield LK, Tweed SO, Petrides B, Swane I (2012) Constraining groundwater flow, residence time, inter-aquifer mixing, and aquifer properties using environmental isotopes in the southeast Murray Basin, Australia. Appl Geochem 27:1698–1709
Celle-Jeanton H, Huneau H, Travi Y, Edmunds WM (2009) Twenty years of groundwater evolution in the Triassic sandstone aquifer of Lorraine: impacts on baseline water quality. Appl Geochem 24:1198–1213
Clark I, Fritz P (1997) Environmental isotopes in hydrology. Lewis, New York, pp 1–328
Coetsiers M, Walraevens K (2009) A new correction model for 14C ages in aquifers with complex geochemistry-application to the Neogene Aquifer, Belgium. Appl Geochem 24:768–776
Coplen TB (1994) Reporting and stable hydrogen, carbon, and oxygen isotopic abundances. Pur Appl Chem 66:273–276
Coplen TB, Wildman JD, Chen J (1991) Improvements in the gaseous hydrogen-water equilibration technique for hydrogen isotope ratio analysis. Anal Chem 63:910–912
Craig H (1961) Isotopic variation in meteoric waters. Science 133:1702–1703
Currell MJ, Cartwright I, Bradley DC, Han D (2010) Recharge history and controls on groundwater quality in the Yuncheng Basin, north China. J Hydrol 385:216–229
Dogramaci SS, Herzceg AL (2002) Strontium and carbon isotope constraints on carbonate-solution interactions and inter-aquifer mixing in groundwaters of the semi-arid Murray Basin, Australia. J Hydrol 262:50–67
Edmunds WM (2009) Geochemistry s vital contribution to solving water resource problems. Appl Geochem 24:1058–1073
Epstein S, Mayeda T (1953) Variations of 18O contents of water from natural sources. Geochim Cosmochim Acta 4:213–224
Gams I, Zupan M (1994) Paka. In: Voglar D (ed) Encyclopedia Slovenia, vol 8 (in Slovene). Mladinska knjiga, Ljubljana, Slovenia, 224 pp
Gieskes JM (1974) The alkalinity-total carbon dioxide system in seawater. In: Goldberg ED (ed) Marine chemistry of the sea. Wiley, New York, pp 123–151
Glavič-Cindro D (2011) Yearly report on gamma and beta ray emiters activity measurements. Working report no. 8/21, Jožef Stefan Institute, Ljubljana, Slovenia, 25 pp
Gonfiantini R (1986) Environmental isotopes in lake studies. Elsevier, Amsterdam, pp 113–168
Gröning M, Rozanski K (2003) Uncertainty assessment of environmental tritium measurements in water. Accred Qual Assur 8:359–366
Hem JD (1985) Study and interpretation of chemical characteristics of natural water. US Geol Surv Water Suppl Pap 2254, 264 pp
Herzeg AI, Leaney FWJ, Stadler MF, Allan GI, Fifield LK (1997) Chemical and isotopic indicators of point-source recharge to a karst aquifer, South Australia. J Hydrol 192:271–299
Kallin RM (2000) Radiocarbon dating of groundwater system. In: Cook P, Herczeg P (eds) Environmental tracers in subsurface. Kluwer, New York, pp 11–144
Kanduč T, Ogrinc N (2007) Hydrogeochemical characteristics of the River Sava watershed in Slovenia. Geologija 50(1):157–177
Kanduč T, Pezdič J (2005) Origin and distribution of coalbed gases from the Velenje Basin, Slovenia. Geochem J 39:397–409
Kanduč T, Szramek K, Ogrinc N, Walter LM (2007) Origin and cycling of riverine inorganic carbon in the Sava River watershed (Slovenia) inferred from major solutes and stable carbon isotopes. Biogeochemistry 86:137–154
Kanduč T, Jamnikar S, McIntosh J (2010) Geochemical characteristics of surface and groundwaters in the Velenje basin (Slovenia). Geologija 53(1):37–46
Kanduč T, Žula J, Zavšek S (2011) Tracing coalbed gas dynamics and origin of gases in advancement of the working faces at mining areas Preloge and Pesje, Velenje Basin. RMZ-Mater Geoenviron 58:273–288
Kanduč T, Markič M, Zavšek S, McIntosh J (2012) Carbon cycling in the Pliocene Velenje Coal Basin, Slovenia, inferred from stable carbon isotopes. Int J Coal Geol 89:70–83
Katz BG, Coplen TB, Bullen TD, Davis JH (1997) Use of chemical and isotopic tracers to characterize the interactions between groundwater and surface water in mantled karst. Ground Water 36(6):1014–1028
Kendall C, Sklash MG, Bullen TD (1995) Isotope tracers of water and solute sources in catchments. In: Trudgill ST (ed) Solute, modeling in catchment system. Wiley, Somerset, NJ, pp 261–303
Kennedy VC, Kendall C, Zellweger GW, Wyerman TA, Avanzino RJ (1986) Determination of the components of stormflow using water chemistry and environmental isotopes, Mattole River Basin, California. J Hydrol 84:107–140
Larsen D, Swihart GH, Xiao Y (2001) Hydrochemistry and isotope composition of springs in the Tecopa basin, southeastern California, USA. Chem Geol 179:17–35
Levin I, Kromer B, Wagenback D, Minnich KO (1987) Carbon isotope measurements of atmospheric CO2 at a coastal station in Antarctica. Tellus 39(B):89–95
Li SL, Liu CQ, Tao FX, Lang YC, Han GL (2005) Carbon biogeochemistry of ground water, Guiyang, Southwest China. Ground Water 43:494–499
Mali N (1992) Uporaba multivariatnih statističnih metod pri ločevanju rudniških vod [The application of multivariate statistical methods to distinguish mining waters]. RMZ 39:347–363
Mali N, Veselič M (1989) Določanje izvora rudniških vod v rudniku lignita Velenje na osnovi njihove kemične sestave [Determination of the origin of mining waters in Velenje coalmine on the basis of their chemical composition]. RMZ 36:383–394
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the central Wasatch Range, Utah. J Hydrol 172:31–59
McIntosh JC, Martini AM (2008) Hydrogeochemical indicators for microbial methane in fractured black shales: case studies of the Antrim, New Albany, and Ohio shales. In: Hill D, Lillis P, Curtis J (eds) Gas shale in the Rocky Mountains and beyond. Association of Geologists 2008 Guidebook, Association of Geologists, Denver, CO, pp 162–174
Mihelak V (2010) Premogovnik Velenje MEJNIKI [Velenje coalmine MILESTONES]. Založnik Premogovnik Velenje, Velenj, Slovenia, 115 pp
Mioč P, Žnidarčič M (1972) Osnovna geološka karta SFRJ 1: 100000 [Basic geological map SFRJ 1:100000]. Tolmač lista Slovenj Gradec [The interpreter sheet Slovenj Gradec], L33–34, Zvezni geološki zavod Beograd, Belgrade, Serbia, 39 pp
Mook WG, Bommerson JC, Staverman WH (1974) Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci Lett 22:169–176
Orem WH, Voytek MA, Jones EJ, Lerch HE, Bates AL, Corum MD, Warwick PD, Clark AC (2010) Organic intermediates in the anaerobic biodegradation of coal to methane under laboratory conditions. Org Geochem 41:997–1000
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol Surv Water Resour Invest Rep 99–4259
Pezdič J (2003) Isotope fractionation of long-term precipitation averages in Ljubljana (Slovenia). RMZ-Mater Geoenviron 50:643–652
Plastino W, Chereji I, Cuna S, Kaihola L, Felice P, Lupsa N, Balas G, Mirel V, Berdea P, Baciu C (2007) Tritium in water electrolytic enrichment and liquid scintillation counting. Radiat Meas 42:68–73
Ranzinger M (2003) X-ray diffraction of clays in the Velenje coalmine (in Slovene). Project work, Velenje coalmine, Velenje, Slovenia, 16 pp
Romanek CS, Grossman EL, Morse JW (1992) Carbon isotopic fractionation in synthetic aragonite and calcite: effects temperature and precipitation rate. Geochim Cosmochim Acta 46:419–430
Šlejkovec Z, Kanduč T (2005) Unexpected arsenic compounds in low-rank coals. Environ Sci Technol 39:3450–3454
Spötl C (2005) A robust and fast method of sampling and analysis of δ13C of dissolved inorganic carbon in groundwaters. Isot Environ Health Stud 41:217–221
Supovec I, Lenart M, Božič D (2012a) Verifikacija vpliva odvodnjevanja triadnih vodonosnikov na območju jame Preloge in jame Škale [Verification of the impact of drainage of Triassic aquifers at mining areas Preloge and Škale], Raziskovalno-razvojna naloga [research and development task]. HGEM, Ljubljana, Slovenia, 28 pp
Supovec I, Lenart M, Božič D (2012b) Analiza vpliva posedanja krovninskih peskov zaradi odkopavanja na uspešnost odvodnjavanja [Analysis of the impact of subsidence of overburden sands due to excavation on success of dewatering], Raziskovalno-razvojna naloga [Research and development task]. HGEM, Ljubljana, Slovenia, 25 pp
Szramek K, Walter LM, Kanduč T, Ogrinc N (2011) Dolomite versus calcite weathering in hydrogeochemically diverse watersheds established on bedded carbonates (Sava and Soča rivers, Slovenia). Aquat Geochem 17:357–396
Taylor CB, Brown LJ, Cunliffe JJ, Davidson PW (1992) Environmental tritium and 18O in a hydrological study of the Wairau Plain and its contributing mountain catchments, Marlborough, New Zeeland. J Hydrol 138:269–319
Urbanc J, Lajlar B (2002) Interpretation of groundwater origin in the Velenje coal mine on the basis of isotope composition (in Slovene). Geologija 45:595–598
Veselič M, Pezdič J (1998) Hydrogeological aspects of lignite mine Velenje: environmental isotope study. RMZ 45:192–196
Villa M, Mannjón G (2004) Low-level measurements of tritium in water. Appl Radiat Isot 61:319–323
Vižintin G, Veselič M, Bombač A, Dervarič E, Likar J, Vukelič Ž (2009) The development of a “drive-in” filters dewatering system in the Velenje coal mine using finite-element modeling. Acta Geotech Slov 1:51–63
Weaver TK, Frape SK, Cherry JA (1995) Recent cross-formational fluid flow and mixing in the shallow Michigan Basin. Geol Soc Am Bull 107:697–707
Williams EL, Szramek KJ, Jin L (2007) The carbonate system geochemistry of shallow groundwater-surface water systems in temperate glaciated watersheds (Michigan, USA): significance of open-system dolomite weathering. Geol Soc Am Bull 119:515–528
Zavadlav S, Kanduč T, McIntosh J, Lojen S (2013) Isotopic and chemical constraints on the biogeochemistry of dissolved Inorganic carbon and chemical weathering in the Karst Watershed of Krka River (Slovenia). Aquat Geochemistry. doi:10.1007/s10498-013-9188-5
Acknowledgements
This study was financially supported by the projects Z1-2052 and L1-5451 funded by the Slovenian Research Agency (ARRS) and the Velenje coalmine D.D. The authors are grateful to Mr. Igor Medved, Mr. Stojan Žigon and Mr. Robert Lah for technical support and assistance with field sampling and analytical help. The authors are grateful to Mrs. Barbara Svetek for 3H measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1057 kb)
Rights and permissions
About this article
Cite this article
Kanduč, T., Grassa, F., McIntosh, J. et al. A geochemical and stable isotope investigation of groundwater/surface-water interactions in the Velenje Basin, Slovenia. Hydrogeol J 22, 971–984 (2014). https://doi.org/10.1007/s10040-014-1103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-014-1103-7